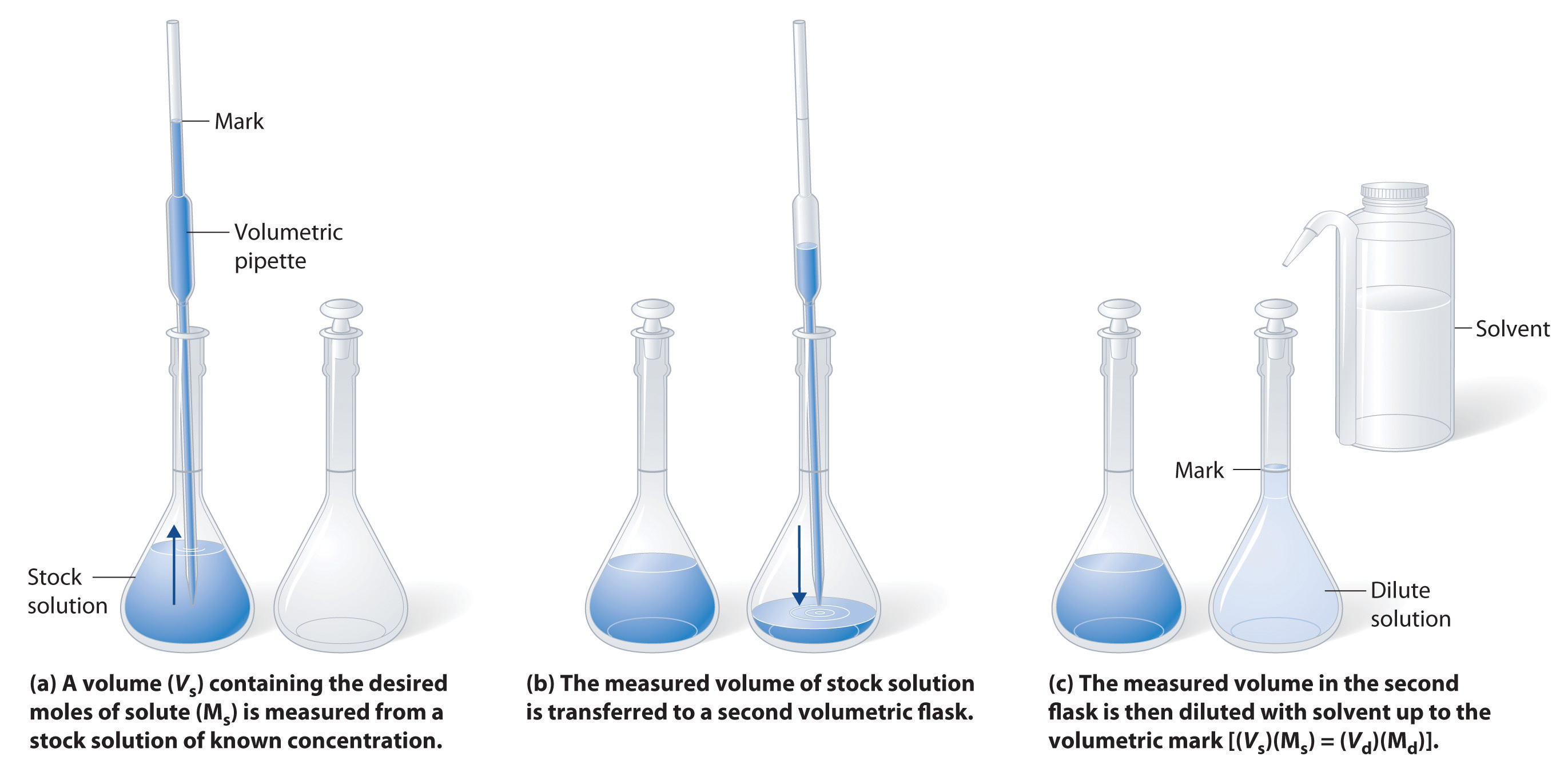
Dilution In Chemistry Terms . Dilution is a process used to lower the concentration of the original solution by adding more solvent. We will begin our discussion of solution concentration with two related and relative terms: The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. A dilute solution is one. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Of course, the resulting solution is thoroughly mixed so as to. To dilute a solution means to add more solvent without the addition of more solute.
from chem.libretexts.org
To dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is thoroughly mixed so as to. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one. The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. Dilution is a process used to lower the concentration of the original solution by adding more solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of. We will begin our discussion of solution concentration with two related and relative terms:
5.2 Solutions and Dilutions Chemistry LibreTexts
Dilution In Chemistry Terms Dilution is a process used to lower the concentration of the original solution by adding more solvent. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Of course, the resulting solution is thoroughly mixed so as to. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of. A dilute solution is one. We will begin our discussion of solution concentration with two related and relative terms: The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. To dilute a solution means to add more solvent without the addition of more solute.
From zhtutorials.com
Serial Dilution Practical Skills Ep 3 Zoë Huggett Tutorials Dilution In Chemistry Terms We will begin our discussion of solution concentration with two related and relative terms: Concentration is the removal of. Dilution is a process used to lower the concentration of the original solution by adding more solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. Of course, the resulting solution is thoroughly mixed so. Dilution In Chemistry Terms.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution In Chemistry Terms State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilute solution is one. Of course, the resulting solution is thoroughly mixed so as to. To dilute a solution means to add more solvent without the addition of more solute. Dilution is a process used to lower the concentration of the original solution by. Dilution In Chemistry Terms.
From www.slideserve.com
PPT Making Dilutions & Electrolytes PowerPoint Presentation, free Dilution In Chemistry Terms Of course, the resulting solution is thoroughly mixed so as to. State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition of more solute. Dilution is a process used to lower the concentration of the original solution by adding more solvent. The process. Dilution In Chemistry Terms.
From www.showme.com
Dilution calculation Science, Chemicalreactions, Biology ShowMe Dilution In Chemistry Terms Dilution is a process used to lower the concentration of the original solution by adding more solvent. To dilute a solution means to add more solvent without the addition of more solute. A dilute solution is one. Concentration is the removal of. State whether the concentration of a solution is directly or indirectly proportional to its volume. Of course, the. Dilution In Chemistry Terms.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution In Chemistry Terms A dilute solution is one. The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. Of course, the resulting solution is thoroughly mixed so as to. State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition. Dilution In Chemistry Terms.
From exovgpnzm.blob.core.windows.net
Dilution And Concentration Of Liquid at Nicole Gardner blog Dilution In Chemistry Terms State whether the concentration of a solution is directly or indirectly proportional to its volume. We will begin our discussion of solution concentration with two related and relative terms: The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. To dilute a solution means to add more solvent without the addition of more. Dilution In Chemistry Terms.
From mmerevise.co.uk
Concentrations and Dilutions MME Dilution In Chemistry Terms We will begin our discussion of solution concentration with two related and relative terms: Concentration is the removal of. To dilute a solution means to add more solvent without the addition of more solute. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one. Of course, the resulting solution. Dilution In Chemistry Terms.
From www3.wolframalpha.com
Dilution Calculator WolframAlpha Chemistry Solvers Dilution In Chemistry Terms Dilution is a process used to lower the concentration of the original solution by adding more solvent. A dilute solution is one. The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of. Dilution. Dilution In Chemistry Terms.
From dxogrtxrg.blob.core.windows.net
Dilution Example In Chemistry at Ignacio Alvarez blog Dilution In Chemistry Terms The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. Concentration is the removal of. State whether the concentration of a solution is directly or indirectly proportional to its volume. We will begin our discussion of solution concentration with two related and relative terms: Dilution is the addition of solvent, which decreases the. Dilution In Chemistry Terms.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution In Chemistry Terms Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one. To dilute a solution means to add more solvent without the addition of more solute. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Of course,. Dilution In Chemistry Terms.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilution In Chemistry Terms To dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is thoroughly mixed so as to. The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is. Dilution In Chemistry Terms.
From www.pinterest.cl
Dilution when solvent is added to dilute a solution, the number of Dilution In Chemistry Terms Concentration is the removal of. State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition of more solute. A dilute solution is one. Of course, the resulting solution is thoroughly mixed so as to. Dilution is the addition of solvent, which decreases the. Dilution In Chemistry Terms.
From www.youtube.com
VA06 Dilution and Aliquot Calculations Year 12 WACE Chemistry YouTube Dilution In Chemistry Terms Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We will begin our discussion of solution concentration with two related and relative terms: Concentration is the removal of. A dilute solution is one. To dilute a solution means to add more solvent without the addition of more solute. Dilution is a process used. Dilution In Chemistry Terms.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution In Chemistry Terms Of course, the resulting solution is thoroughly mixed so as to. A dilute solution is one. To dilute a solution means to add more solvent without the addition of more solute. Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution is. Dilution In Chemistry Terms.
From exopkbipv.blob.core.windows.net
Dilution Equation Example Chemistry at Wanda King blog Dilution In Chemistry Terms We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Concentration is the removal of. The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. To dilute. Dilution In Chemistry Terms.
From www.pinterest.com
Dilute Flashcards, Easy science, Science student Dilution In Chemistry Terms Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. To dilute a solution means to add more solvent without the addition of more solute. We will begin our discussion of solution concentration with two related and relative terms: Of course, the resulting solution is thoroughly mixed so as to. The process increases the. Dilution In Chemistry Terms.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution In Chemistry Terms To dilute a solution means to add more solvent without the addition of more solute. We will begin our discussion of solution concentration with two related and relative terms: Concentration is the removal of. Dilution is a process used to lower the concentration of the original solution by adding more solvent. A dilute solution is one. The process increases the. Dilution In Chemistry Terms.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Dilution In Chemistry Terms A dilute solution is one. We will begin our discussion of solution concentration with two related and relative terms: Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Of course, the resulting solution is thoroughly mixed. Dilution In Chemistry Terms.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution In Chemistry Terms Concentration is the removal of. State whether the concentration of a solution is directly or indirectly proportional to its volume. Of course, the resulting solution is thoroughly mixed so as to. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the concentration of the original solution. Dilution In Chemistry Terms.
From www.labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution In Chemistry Terms A dilute solution is one. We will begin our discussion of solution concentration with two related and relative terms: Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. Of course, the. Dilution In Chemistry Terms.
From chemistnotes.com
Isotopic Dilution Method Detailed Explanation Chemistry Notes Dilution In Chemistry Terms Concentration is the removal of. To dilute a solution means to add more solvent without the addition of more solute. A dilute solution is one. State whether the concentration of a solution is directly or indirectly proportional to its volume. We will begin our discussion of solution concentration with two related and relative terms: The process increases the total volume. Dilution In Chemistry Terms.
From www.answersarena.com
[Solved] Dilution diagrams can be very helpful in organiz Dilution In Chemistry Terms The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. We will begin our discussion of solution concentration with two related and relative terms: Of course, the resulting solution is thoroughly mixed so as to. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is. Dilution In Chemistry Terms.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Dilution In Chemistry Terms Concentration is the removal of. State whether the concentration of a solution is directly or indirectly proportional to its volume. The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Of course, the resulting solution is. Dilution In Chemistry Terms.
From www.youtube.com
Beginning Chemistry Solution Dilution YouTube Dilution In Chemistry Terms Of course, the resulting solution is thoroughly mixed so as to. The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. Concentration is the removal of. We will begin our discussion of solution concentration with two related and relative terms: Dilution is the addition of solvent, which decreases the concentration of the solute. Dilution In Chemistry Terms.
From carlosgokeowen.blogspot.com
What is Dilution Dilution In Chemistry Terms The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. Dilution is a process used to lower the concentration of the original solution by adding more solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilute solution is one. Of course, the resulting solution is. Dilution In Chemistry Terms.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution In Chemistry Terms State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition of more solute. A dilute solution is one. Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We will begin our. Dilution In Chemistry Terms.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution In Chemistry Terms The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. To dilute a solution means to add more solvent without the addition of more solute. We will begin our discussion of solution concentration with two related and relative terms: Of course, the resulting solution is thoroughly mixed so as to. Dilution is a. Dilution In Chemistry Terms.
From exovgpnzm.blob.core.windows.net
Dilution And Concentration Of Liquid at Nicole Gardner blog Dilution In Chemistry Terms Concentration is the removal of. The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. Of course, the resulting solution is thoroughly mixed so as to. Dilution is the addition of solvent, which decreases. Dilution In Chemistry Terms.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution In Chemistry Terms Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of. Of course, the resulting solution is thoroughly mixed so as to. A dilute solution is one. The process increases the total volume of the. Dilution In Chemistry Terms.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution In Chemistry Terms Of course, the resulting solution is thoroughly mixed so as to. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. Dilution is a process used to lower the concentration of the original solution by adding more. Dilution In Chemistry Terms.
From www.youtube.com
What is Dilute Solution? Chemistry YouTube Dilution In Chemistry Terms Concentration is the removal of. Of course, the resulting solution is thoroughly mixed so as to. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. The process increases the total volume of the solution while proportionally. Dilution In Chemistry Terms.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution In Chemistry Terms To dilute a solution means to add more solvent without the addition of more solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilute solution is one. The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. Dilution is a process used to lower the. Dilution In Chemistry Terms.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution In Chemistry Terms The process increases the total volume of the solution while proportionally decreasing the concentration of the solute. To dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is thoroughly mixed so as to. A dilute solution is one. State whether the concentration of a solution is directly or indirectly proportional. Dilution In Chemistry Terms.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution In Chemistry Terms A dilute solution is one. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We will begin our discussion of solution concentration with two related and relative terms: Of course, the resulting solution is thoroughly mixed. Dilution In Chemistry Terms.
From www.youtube.com
Chem143 Dilution Equation YouTube Dilution In Chemistry Terms Of course, the resulting solution is thoroughly mixed so as to. A dilute solution is one. Concentration is the removal of. We will begin our discussion of solution concentration with two related and relative terms: To dilute a solution means to add more solvent without the addition of more solute. The process increases the total volume of the solution while. Dilution In Chemistry Terms.