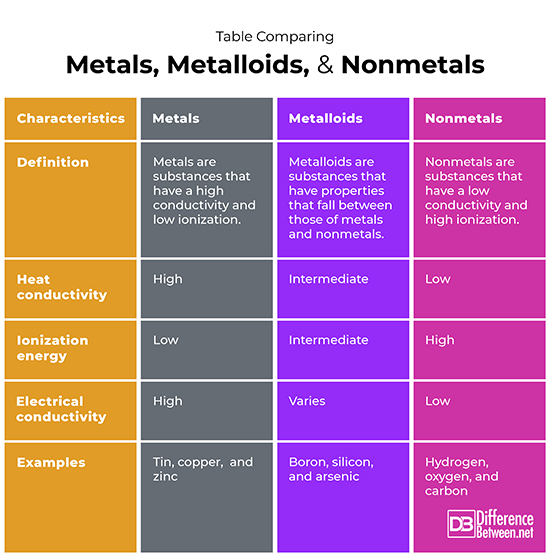
Examples Are Metalloids . On the periodic table, the. While metalloids are neither good electrical nor thermal conductors, they make excellent semiconductors and form amphoteric oxides. Metalloids are used to make semiconductors, ceramics, polymers, and batteries. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. Take a closer look at the list of metalloids, their properties, and uses. Metalloids can also be called semimetals. Metalloids exhibit remarkable versatility due to their distinctive properties, rendering them highly advantageous for a diverse array of applications encompassing. Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements.
from www.differencebetween.net
Metalloids exhibit remarkable versatility due to their distinctive properties, rendering them highly advantageous for a diverse array of applications encompassing. On the periodic table, the. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. Metalloids are used to make semiconductors, ceramics, polymers, and batteries. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. While metalloids are neither good electrical nor thermal conductors, they make excellent semiconductors and form amphoteric oxides. These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. Take a closer look at the list of metalloids, their properties, and uses.
Difference Between Metals, Metalloids, and Nonmetals Difference Between
Examples Are Metalloids Metalloids can also be called semimetals. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Take a closer look at the list of metalloids, their properties, and uses. These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. On the periodic table, the. Metalloids exhibit remarkable versatility due to their distinctive properties, rendering them highly advantageous for a diverse array of applications encompassing. Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. Metalloids are used to make semiconductors, ceramics, polymers, and batteries. Metalloids can also be called semimetals. While metalloids are neither good electrical nor thermal conductors, they make excellent semiconductors and form amphoteric oxides.
From blog.thepipingmart.com
Metalloids Uses and Properties Examples Are Metalloids Take a closer look at the list of metalloids, their properties, and uses. Metalloids are used to make semiconductors, ceramics, polymers, and batteries. While metalloids are neither good electrical nor thermal conductors, they make excellent semiconductors and form amphoteric oxides. Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or. Examples Are Metalloids.
From www.differencebetween.net
Difference Between Metals, Metalloids, and Nonmetals Difference Between Examples Are Metalloids On the periodic table, the. Take a closer look at the list of metalloids, their properties, and uses. While metalloids are neither good electrical nor thermal conductors, they make excellent semiconductors and form amphoteric oxides. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. The elements boron, silicon, germanium, arsenic, antimony, and. Examples Are Metalloids.
From www.slideserve.com
PPT Metals, Nonmetals and Metalloids PowerPoint Presentation, free Examples Are Metalloids A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. Metalloids are used to make semiconductors, ceramics, polymers, and batteries. Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when. Examples Are Metalloids.
From www.slideserve.com
PPT Metals, Metalloids, and Nonmetals PowerPoint Presentation, free Examples Are Metalloids Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements. Metalloids are used to make semiconductors, ceramics, polymers, and batteries. On the periodic table, the. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Metalloids exhibit. Examples Are Metalloids.
From thechemistrynotes.com
Metalloids Definition, Properties, Uses, and Applications Examples Are Metalloids The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. While metalloids are neither good electrical nor thermal conductors, they make excellent semiconductors and form amphoteric oxides. Metalloids can also be called semimetals. Metalloids. Examples Are Metalloids.
From sciencetrends.com
4 Properties Of Metalloids Science Trends Examples Are Metalloids Metalloids can also be called semimetals. These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. While metalloids are neither good electrical nor thermal conductors, they make excellent semiconductors and form amphoteric oxides. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Metalloids tend to be shiny,. Examples Are Metalloids.
From testbook.com
Metalloids Learn its Definition, Examples, Properties and Uses Examples Are Metalloids On the periodic table, the. While metalloids are neither good electrical nor thermal conductors, they make excellent semiconductors and form amphoteric oxides. Metalloids can also be called semimetals. Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements. Metalloids exhibit remarkable versatility due to their distinctive. Examples Are Metalloids.
From www.teachoo.com
Metals, Non Metals and Metalloids Meaning & Difference Teachoo Examples Are Metalloids The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. These elements, called metalloids or. Examples Are Metalloids.
From www.haikudeck.com
Metalloids by Megan Maul Examples Are Metalloids Metalloids can also be called semimetals. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. While metalloids are neither good electrical nor thermal conductors, they make excellent semiconductors and form amphoteric oxides. Take a closer. Examples Are Metalloids.
From brokeasshome.com
Metalloids Located On The Periodic Table Examples Are Metalloids A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Take a closer look at the list of metalloids, their properties, and uses. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. While metalloids are neither good electrical nor thermal conductors, they make. Examples Are Metalloids.
From scienceinfo.com
Metalloids Definition, Properties, Uses, and Applications Examples Are Metalloids Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. Metalloids can also be. Examples Are Metalloids.
From sciencenotes.org
List of Metalloids or Semimetals Examples Are Metalloids A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. Take a closer look at. Examples Are Metalloids.
From www.chemistrylearner.com
Metalloids Chemistry Learner Examples Are Metalloids These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. On the periodic table, the. Metalloids can also be called semimetals. Metalloids exhibit remarkable versatility due to their distinctive properties, rendering them highly advantageous for a diverse array of applications encompassing. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the. Examples Are Metalloids.
From newtondesk.com
Metalloids (Periodic Table) Properties, Uses, & Facts Examples Are Metalloids Metalloids are used to make semiconductors, ceramics, polymers, and batteries. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. While metalloids are neither good electrical nor thermal conductors, they make excellent semiconductors and form amphoteric oxides. Take a closer look at the list of metalloids, their properties, and uses. On the periodic. Examples Are Metalloids.
From www.geeksforgeeks.org
Metalloids Definition, Position in Periodic Table, & Properties Examples Are Metalloids Metalloids can also be called semimetals. Metalloids are used to make semiconductors, ceramics, polymers, and batteries. Take a closer look at the list of metalloids, their properties, and uses. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. A metalloid is an element that has properties that are intermediate between. Examples Are Metalloids.
From periodictableguide.com
Where are Metalloids located on the Periodic table? (Images) Examples Are Metalloids Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. Take a closer look at the list of metalloids, their properties, and uses. Metalloids can also be called semimetals. Metalloids exhibit remarkable versatility due to their. Examples Are Metalloids.
From www.slideserve.com
PPT ELEMENT CLASSES PowerPoint Presentation ID149914 Examples Are Metalloids On the periodic table, the. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements. Metalloids exhibit remarkable versatility due to their distinctive properties, rendering them highly. Examples Are Metalloids.
From www.slideserve.com
PPT PERIODIC TABLE PowerPoint Presentation, free download ID6893850 Examples Are Metalloids Metalloids are used to make semiconductors, ceramics, polymers, and batteries. Metalloids exhibit remarkable versatility due to their distinctive properties, rendering them highly advantageous for a diverse array of applications encompassing. On the periodic table, the. Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements. Take. Examples Are Metalloids.
From utedzz.blogspot.com
Periodic Table Metalloids Periodic Table Timeline Examples Are Metalloids These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. Metalloids are used to make semiconductors, ceramics, polymers, and batteries. Metalloids can also be called semimetals. Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements. Take a closer look at. Examples Are Metalloids.
From www.difference101.com
Metals vs. Nonmetals vs. Metalloids 5 Key Differences, Pros & Cons Examples Are Metalloids Metalloids exhibit remarkable versatility due to their distinctive properties, rendering them highly advantageous for a diverse array of applications encompassing. These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. On the periodic table, the. While metalloids are neither good electrical nor thermal conductors, they make excellent semiconductors and form amphoteric oxides. Take a closer. Examples Are Metalloids.
From www.xometry.com
Metalloids Properties and Uses Xometry Examples Are Metalloids Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Metalloids are used to make semiconductors, ceramics, polymers, and batteries. These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. Take a closer look at the list of metalloids, their properties, and uses. Metalloids tend to. Examples Are Metalloids.
From www.adda247.com
What are Metalloids? Definition, Properties and Example Examples Are Metalloids A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Take a closer look at the list of metalloids, their properties, and uses. Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements. Metalloids are used to make semiconductors,. Examples Are Metalloids.
From pediabay.com
Metalloids of the Periodic Table Pediabay Examples Are Metalloids Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements. On the periodic table, the. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals. Examples Are Metalloids.
From www.worksheetsplanet.com
What are Metalloids? Examples Are Metalloids Metalloids can also be called semimetals. On the periodic table, the. Take a closer look at the list of metalloids, their properties, and uses. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. While metalloids are neither. Examples Are Metalloids.
From sciencenotes.org
5 Examples of Metals, Metalloids, and Nonmetals Examples Are Metalloids Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements. While metalloids are neither good electrical nor thermal conductors, they make excellent semiconductors and form amphoteric oxides. Metalloids exhibit remarkable versatility due to their distinctive properties, rendering them highly advantageous for a diverse array of applications. Examples Are Metalloids.
From www.breakingatom.com
Metalloid Definition Examples Are Metalloids These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. On the periodic table, the. Metalloids can also be called semimetals. While metalloids are neither good electrical nor thermal conductors, they make excellent semiconductors and form amphoteric oxides. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance. Examples Are Metalloids.
From www.youtube.com
Metalloids YouTube Examples Are Metalloids These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. On the periodic table, the. Metalloids are used to make semiconductors, ceramics, polymers, and batteries. Metalloids can also be called semimetals. While metalloids are neither good. Examples Are Metalloids.
From newtondesk.com
Metalloids (Periodic Table) Properties, Uses, & Facts NewtonDesk Examples Are Metalloids Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements. While metalloids are neither good electrical nor thermal conductors, they make excellent semiconductors and form amphoteric oxides. Take a closer look at the list of metalloids, their properties, and uses. Metalloid, in chemistry, an imprecise term. Examples Are Metalloids.
From periodictableguide.com
Periodic table labeled with Metals Nonmetals and Metalloids Examples Are Metalloids Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. These elements, called metalloids or. Examples Are Metalloids.
From ck12.org
Groups with Metalloids CK12 Foundation Examples Are Metalloids Metalloids can also be called semimetals. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when. Examples Are Metalloids.
From study.com
Metalloid Elements Definition, Properties & Examples Lesson Examples Are Metalloids Metalloids can also be called semimetals. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. On the periodic table, the. Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements. These elements, called metalloids or sometimes. Examples Are Metalloids.
From www.youtube.com
Definition of metalloids for class 8 science. YouTube Examples Are Metalloids Metalloids can also be called semimetals. Metalloids exhibit remarkable versatility due to their distinctive properties, rendering them highly advantageous for a diverse array of applications encompassing. Metalloids are used to make semiconductors, ceramics, polymers, and batteries. Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements.. Examples Are Metalloids.
From ar.inspiredpencil.com
Metalloids Examples Examples Are Metalloids On the periodic table, the. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Metalloids are used to make semiconductors, ceramics, polymers, and batteries. Metalloids can also be called semimetals. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. The elements boron,. Examples Are Metalloids.
From knordslearning.com
Metalloids Periodic Table (With Images) Examples Are Metalloids A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Metalloids are used to make semiconductors, ceramics, polymers, and batteries. On the periodic table, the. Metalloid, in chemistry, an imprecise term used to describe a chemical element that forms a simple substance having properties. Metalloids exhibit remarkable versatility due to their distinctive properties,. Examples Are Metalloids.
From sciencenotes.org
Metalloids Science Notes and Projects Examples Are Metalloids Metalloids are used to make semiconductors, ceramics, polymers, and batteries. These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. On the periodic table, the. Metalloid, in chemistry, an imprecise term used to describe a chemical element that. Examples Are Metalloids.