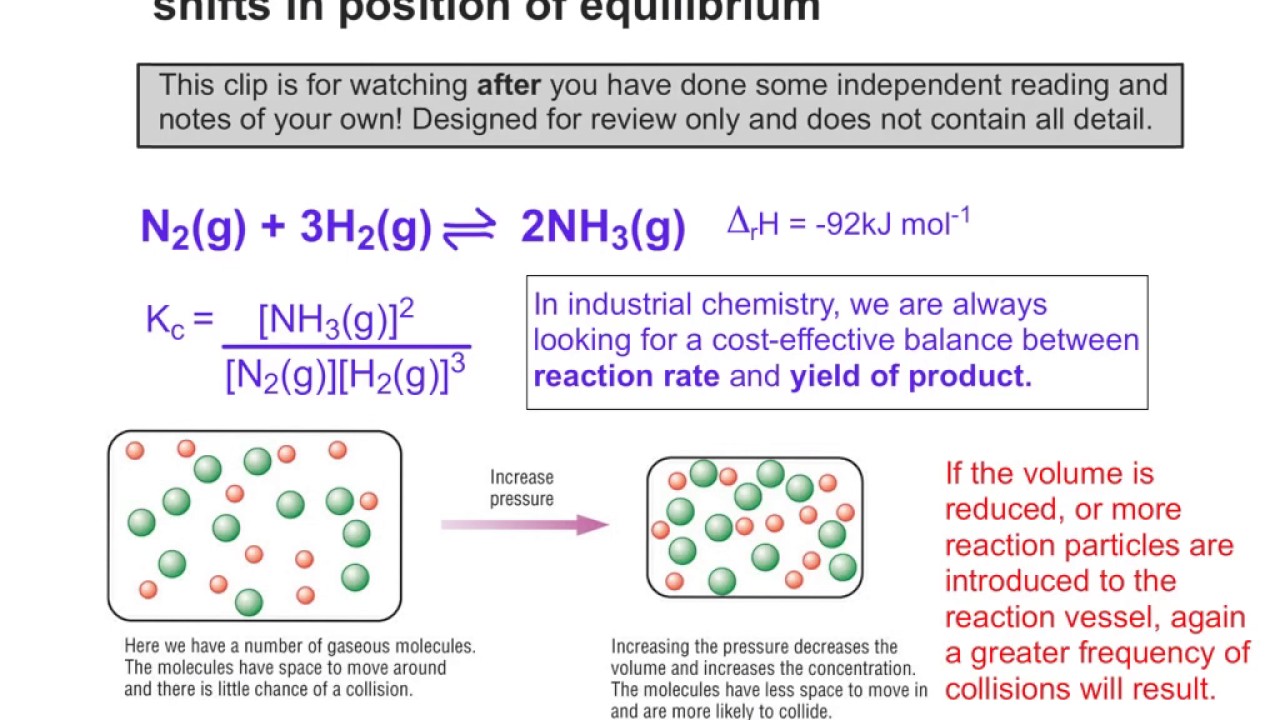
What Does Shift Right Mean In Equilibrium . Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or. Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. The direction of shift can be predicted for changes in concentrations, temperature,. In accordance with le châtelier's principle, a shift in the equilibrium that reduces the total number of molecules per unit of volume will be favored. If an equilibrium system is. Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the equilibrium itself. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting.
from www.youtube.com
Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. In accordance with le châtelier's principle, a shift in the equilibrium that reduces the total number of molecules per unit of volume will be favored. A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the equilibrium itself. If an equilibrium system is. The direction of shift can be predicted for changes in concentrations, temperature,. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting.
Quick review equilibrium shifts explained YouTube
What Does Shift Right Mean In Equilibrium The direction of shift can be predicted for changes in concentrations, temperature,. If an equilibrium system is. Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The direction of shift can be predicted for changes in concentrations, temperature,. Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the equilibrium itself. In accordance with le châtelier's principle, a shift in the equilibrium that reduces the total number of molecules per unit of volume will be favored.
From mmerevise.co.uk
Reversible Reactions and Equilibrium Revision MME What Does Shift Right Mean In Equilibrium If an equilibrium system is. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or. In accordance with le châtelier's principle, a shift in the equilibrium that reduces the total number. What Does Shift Right Mean In Equilibrium.
From www.slideshare.net
(Chapter 7) And Equilibrium What Does Shift Right Mean In Equilibrium Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the equilibrium itself. Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. A chemical system at equilibrium can be. What Does Shift Right Mean In Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Does Shift Right Mean In Equilibrium Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The direction of shift can be predicted for changes in concentrations, temperature,. If an equilibrium system is. In accordance with le châtelier's principle, a shift in the equilibrium that. What Does Shift Right Mean In Equilibrium.
From saylordotorg.github.io
Demand, Supply, and Equilibrium What Does Shift Right Mean In Equilibrium A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or. If an equilibrium system is. In accordance with le châtelier's principle, a shift in the equilibrium that reduces the total number of molecules per unit of volume will be favored. Consequently, changes in concentration and temperature are. What Does Shift Right Mean In Equilibrium.
From www.slideserve.com
PPT 61 Seeking Equilibrium Demand and Supply PowerPoint What Does Shift Right Mean In Equilibrium Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Beverages are exposed to a high pressure of gaseous carbon dioxide during the. What Does Shift Right Mean In Equilibrium.
From www.slideserve.com
PPT Le Châtelier’s Principle PowerPoint Presentation, free download What Does Shift Right Mean In Equilibrium If an equilibrium system is. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. The direction of shift can be predicted for changes in concentrations, temperature,. In accordance with le. What Does Shift Right Mean In Equilibrium.
From www.tutor2u.net
Changes in Market Equilibrium Price tutor2u Economics What Does Shift Right Mean In Equilibrium Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. In accordance with le châtelier's principle, a shift in the equilibrium that reduces the total number of molecules per unit of volume will be favored. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above. What Does Shift Right Mean In Equilibrium.
From www.thetutoracademy.com
Equilibrium Levels of Real National Output The Tutor Academy What Does Shift Right Mean In Equilibrium A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or. Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. In accordance with le châtelier's principle,. What Does Shift Right Mean In Equilibrium.
From www.youtube.com
Easily Remember the Things that Shift the Demand Curve YouTube What Does Shift Right Mean In Equilibrium Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. In accordance with le châtelier's principle, a shift in the equilibrium that reduces the total number of molecules per unit of volume will be favored. If an equilibrium system is. Simply it means that the equilibrium will force the conversion of certain substances to. What Does Shift Right Mean In Equilibrium.
From www.slideserve.com
PPT Chapter 3 Market Equilibrium PowerPoint Presentation, free What Does Shift Right Mean In Equilibrium The direction of shift can be predicted for changes in concentrations, temperature,. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. In accordance with le châtelier's principle, a shift in the equilibrium that reduces the total number of molecules per unit of volume will be favored. A chemical system at equilibrium can be. What Does Shift Right Mean In Equilibrium.
From www.slideserve.com
PPT Module 7 Changes in Equilibrium PowerPoint Presentation ID1557169 What Does Shift Right Mean In Equilibrium Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the equilibrium itself. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The direction of shift can be. What Does Shift Right Mean In Equilibrium.
From tutorstips.com
Movement Along Supply Curve and Shift in Supply Curve Tutor's Tips What Does Shift Right Mean In Equilibrium Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the equilibrium. What Does Shift Right Mean In Equilibrium.
From medlifemastery.com
Equilibrium on the MCAT MedLife Mastery What Does Shift Right Mean In Equilibrium Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The direction of shift can be predicted for changes in concentrations, temperature,. A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or. Le chatelier's principle addresses how an equilibrium shifts when. What Does Shift Right Mean In Equilibrium.
From www.youtube.com
Le Chatelier's Principle and Equilibrium Shifts YouTube What Does Shift Right Mean In Equilibrium A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. If an equilibrium system is. Simply it means that the equilibrium will force the conversion of certain substances to reduce the. What Does Shift Right Mean In Equilibrium.
From slidetodoc.com
Changes in Equilibrium Lesson 2 7 Changes in What Does Shift Right Mean In Equilibrium If an equilibrium system is. The direction of shift can be predicted for changes in concentrations, temperature,. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. Le chatelier's principle addresses how an equilibrium shifts when the conditions of. What Does Shift Right Mean In Equilibrium.
From kenzierosbrady.blogspot.com
Supply Curve Shift Right KenzierosBrady What Does Shift Right Mean In Equilibrium Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the equilibrium itself. If an equilibrium system is. A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or. The direction of shift can be predicted for changes in. What Does Shift Right Mean In Equilibrium.
From www.thebalancemoney.com
What Does It Mean When There's a Shift in Demand Curve? What Does Shift Right Mean In Equilibrium Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. In accordance with le châtelier's principle, a shift in the equilibrium that reduces the total number of molecules per unit of volume will be favored. The direction of shift can be predicted for changes in concentrations, temperature,. Simply it means that the equilibrium will. What Does Shift Right Mean In Equilibrium.
From articles.outlier.org
Understanding the Supply Curve & How It Works Outlier What Does Shift Right Mean In Equilibrium Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the equilibrium itself. In accordance with le châtelier's principle, a shift in the equilibrium that reduces the total number of molecules per unit of volume. What Does Shift Right Mean In Equilibrium.
From articles.outlier.org
Overview of Movement vs. Shift in the Demand Curve Outlier What Does Shift Right Mean In Equilibrium Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the. What Does Shift Right Mean In Equilibrium.
From www.slideserve.com
PPT Chapter 3 Market Equilibrium PowerPoint Presentation, free What Does Shift Right Mean In Equilibrium If an equilibrium system is. The direction of shift can be predicted for changes in concentrations, temperature,. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the equilibrium itself. In accordance with le châtelier's. What Does Shift Right Mean In Equilibrium.
From www.learncram.com
Shifts in Demand and Supply Decrease and Increase, Concepts, Examples What Does Shift Right Mean In Equilibrium Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the equilibrium itself. The direction of shift can be predicted for changes in concentrations, temperature,. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Consequently, changes in concentration and temperature are the two stresses. What Does Shift Right Mean In Equilibrium.
From spureconomics.com
Market Equilibrium, Shifts and Role of Elasticity What Does Shift Right Mean In Equilibrium Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. If an equilibrium system is. Simply it means that the equilibrium will force the conversion of certain substances to reduce. What Does Shift Right Mean In Equilibrium.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 What Does Shift Right Mean In Equilibrium The direction of shift can be predicted for changes in concentrations, temperature,. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing. What Does Shift Right Mean In Equilibrium.
From www.vrogue.co
Chemical Equilibrium Definition Types Importance And vrogue.co What Does Shift Right Mean In Equilibrium Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Beverages are exposed to a high. What Does Shift Right Mean In Equilibrium.
From articles.outlier.org
Predicting Changes in Equilibrium Price and Quantity Outlier What Does Shift Right Mean In Equilibrium Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. In accordance with le châtelier's principle, a shift in the equilibrium that reduces the total number of molecules per unit of volume will be favored. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The direction of shift. What Does Shift Right Mean In Equilibrium.
From www.youtube.com
Quick review equilibrium shifts explained YouTube What Does Shift Right Mean In Equilibrium A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or. In accordance with le châtelier's principle, a shift in the equilibrium that reduces the total number of molecules per unit of volume will be favored. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of. What Does Shift Right Mean In Equilibrium.
From www.slideserve.com
PPT Le Châtelier’s Principle PowerPoint Presentation, free download What Does Shift Right Mean In Equilibrium A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or. The direction of shift can be predicted for changes in concentrations, temperature,. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. If an equilibrium system is. Consequently, changes in concentration. What Does Shift Right Mean In Equilibrium.
From ilearnthis.com
What is Shift in Demand Curve? Examples & Factors What Does Shift Right Mean In Equilibrium Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the equilibrium itself. In accordance with le châtelier's principle, a shift in the equilibrium that reduces the total number of molecules per unit of volume will be favored. Consequently, changes in concentration and temperature are the two stresses that can shift. What Does Shift Right Mean In Equilibrium.
From www.youtube.com
Reaction Quotient and Equilibrium Shift YouTube What Does Shift Right Mean In Equilibrium Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Le chatelier's principle addresses how an. What Does Shift Right Mean In Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Does Shift Right Mean In Equilibrium The direction of shift can be predicted for changes in concentrations, temperature,. Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the equilibrium itself. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. A chemical. What Does Shift Right Mean In Equilibrium.
From www.youtube.com
Movement Vs Shift in Demand Curve Difference between them with What Does Shift Right Mean In Equilibrium The direction of shift can be predicted for changes in concentrations, temperature,. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or. Consequently,. What Does Shift Right Mean In Equilibrium.
From www.tutor2u.net
Market Equilibrium Transition to New Equilibrium tutor2u Economics What Does Shift Right Mean In Equilibrium Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the equilibrium itself. Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. The direction of shift can be predicted. What Does Shift Right Mean In Equilibrium.
From ilearnthis.com
3 Steps to Analyzing Changes in Equilibrium ilearnthis What Does Shift Right Mean In Equilibrium The direction of shift can be predicted for changes in concentrations, temperature,. Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. If an equilibrium system is. Beverages are exposed to a high pressure of gaseous carbon dioxide during the process to shift the first equilibrium above to the right, resulting. Simply it means that. What Does Shift Right Mean In Equilibrium.
From www.thestudentroom.co.uk
Equilibrium issues with understanding The Student Room What Does Shift Right Mean In Equilibrium Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium. In accordance with le châtelier's principle, a shift in the equilibrium that reduces the total number of molecules per unit of volume will be favored. Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the. What Does Shift Right Mean In Equilibrium.
From www.youtube.com
2.08b Chemical Equilibrium How Changes Shift Equilibrium YouTube What Does Shift Right Mean In Equilibrium Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Simply it means that the equilibrium will force the conversion of certain substances to reduce the disturbance, to maintain the equilibrium itself. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. In accordance with le châtelier's principle,. What Does Shift Right Mean In Equilibrium.