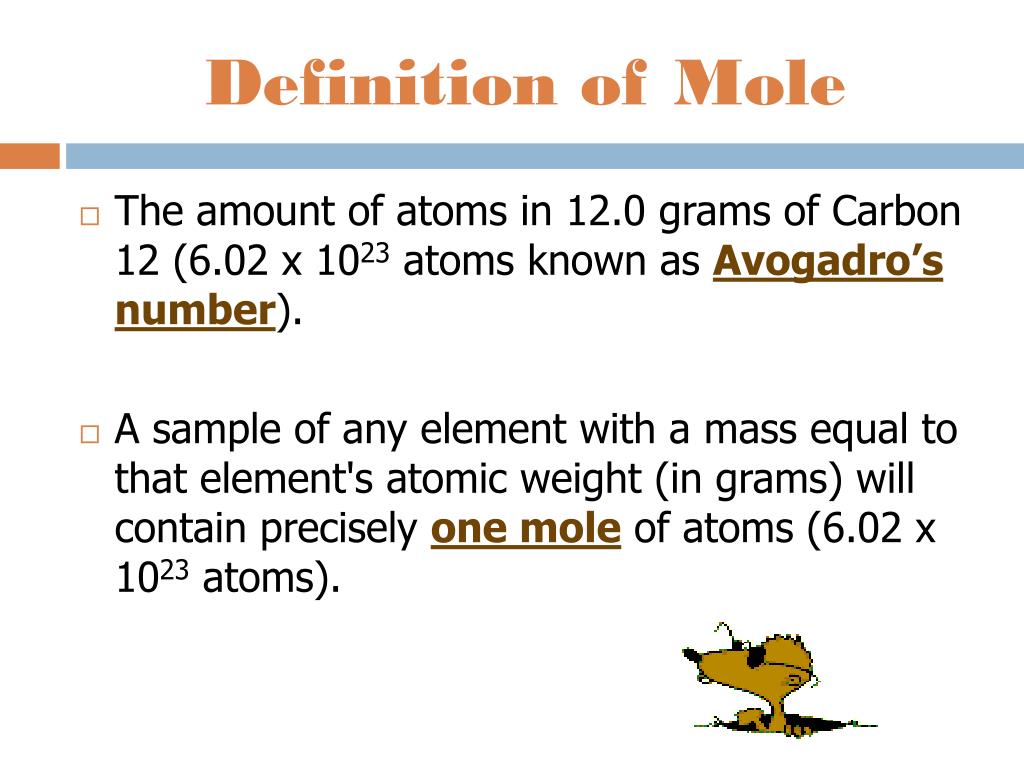
What Does Mole Represent In Chemistry . A mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. Here's a look at what a mole is, why we use moles, and how to convert between moles and grams. 6.02x10 23 is a unique number, called avogadro’s number. A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. It is defined as the amount of a. The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. Updated on july 03, 2019. It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. The mole is the unit of measurement in the international system of units (si) for amount of substance. The term “mole” is defined as the quantity or mass of a substance that contains 6.02 × 10 23 particles (atoms, molecules, or formula units). Mole in chemistry the mole is an si unit used to.
from www.slideserve.com
It is defined as the amount of a. The mole is the unit of measurement in the international system of units (si) for amount of substance. Updated on july 03, 2019. A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. Mole in chemistry the mole is an si unit used to. The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. 6.02x10 23 is a unique number, called avogadro’s number. Here's a look at what a mole is, why we use moles, and how to convert between moles and grams. It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). The term “mole” is defined as the quantity or mass of a substance that contains 6.02 × 10 23 particles (atoms, molecules, or formula units).
PPT Unit 4 The Mole PowerPoint Presentation, free download ID5601061
What Does Mole Represent In Chemistry The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. A mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. 6.02x10 23 is a unique number, called avogadro’s number. Here's a look at what a mole is, why we use moles, and how to convert between moles and grams. It is defined as the amount of a. Mole in chemistry the mole is an si unit used to. The mole is the unit of measurement in the international system of units (si) for amount of substance. Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. Updated on july 03, 2019. The term “mole” is defined as the quantity or mass of a substance that contains 6.02 × 10 23 particles (atoms, molecules, or formula units). It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction.
From sciencenotes.org
What Is a Molecule? Definition and Examples What Does Mole Represent In Chemistry The term “mole” is defined as the quantity or mass of a substance that contains 6.02 × 10 23 particles (atoms, molecules, or formula units). Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. A mole is a unit of measurement that is used to express the amount of substance. What Does Mole Represent In Chemistry.
From studylib.net
THE MOLE Chemistry What Does Mole Represent In Chemistry The mole is the unit of measurement in the international system of units (si) for amount of substance. The term “mole” is defined as the quantity or mass of a substance that contains 6.02 × 10 23 particles (atoms, molecules, or formula units). Updated on july 03, 2019. It is defined that 1 mole of any substance contains 6.02x1023 particles. What Does Mole Represent In Chemistry.
From www.alamy.com
Mole formula, illustration Stock Photo Alamy What Does Mole Represent In Chemistry It is defined as the amount of a. Updated on july 03, 2019. Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. Mole in chemistry the mole is an si unit used to. The term “mole” is defined as the quantity or mass of a substance that contains 6.02 ×. What Does Mole Represent In Chemistry.
From sciencenotes.org
Mole Fraction Formula and Calculation What Does Mole Represent In Chemistry A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. 6.02x10 23 is a unique number, called avogadro’s number. It is defined as the amount of a. Here's a look at what a mole is, why we use moles, and how to convert between moles and grams. A mole is. What Does Mole Represent In Chemistry.
From surfguppy.com
The Mole relationship to Carbon Surfguppy Chemistry made easy What Does Mole Represent In Chemistry The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. Mole in chemistry the mole is an si unit used to. It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such. What Does Mole Represent In Chemistry.
From www.slideserve.com
PPT Unit 4 The Mole PowerPoint Presentation, free download ID5601061 What Does Mole Represent In Chemistry A mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). Here's a look at what a mole is, why we use moles, and how to convert between moles and grams. A mole is a unit of. What Does Mole Represent In Chemistry.
From www.thoughtco.com
What Is a Mole in Chemistry? What Does Mole Represent In Chemistry It is defined as the amount of a. A mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. Updated on july 03, 2019. A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. The mole is the unit of measurement in the. What Does Mole Represent In Chemistry.
From chemistrymadesimple.net
Why the Mole Concept is the MOST IMPORTANT concept in College Chemistry What Does Mole Represent In Chemistry 6.02x10 23 is a unique number, called avogadro’s number. The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. The mole is the unit of measurement in the international system of units (si) for amount of substance. Here's a look at what a mole is, why we use moles, and how to convert. What Does Mole Represent In Chemistry.
From www.pinterest.com
Mole definition The mole is the unit of amount in chemistry. a What Does Mole Represent In Chemistry The mole is the unit of measurement in the international system of units (si) for amount of substance. The term “mole” is defined as the quantity or mass of a substance that contains 6.02 × 10 23 particles (atoms, molecules, or formula units). A mole is a unit of measurement that is used to express the amount of substance in. What Does Mole Represent In Chemistry.
From www.slideserve.com
PPT The Mole Concept PowerPoint Presentation, free download ID4787514 What Does Mole Represent In Chemistry A mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. Updated on july 03, 2019. It is defined as the amount of a. It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). A mole is a unit of measurement that is used to express the. What Does Mole Represent In Chemistry.
From www.showme.com
Basic mole conversions part 1 Science, Chemistry, Moles ShowMe What Does Mole Represent In Chemistry The mole is the unit of measurement in the international system of units (si) for amount of substance. A mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). Here's a look at what a mole is,. What Does Mole Represent In Chemistry.
From www.slideserve.com
PPT The Chemistry Mole PowerPoint Presentation, free download ID What Does Mole Represent In Chemistry A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. The mole is the unit of measurement in the international system of units (si) for amount of substance. It is defined as the amount of a. A mole is defined as a chemical unit, defined to be 6.022 x 10. What Does Mole Represent In Chemistry.
From www.youtube.com
How to Use the MOLE in Chemistry YouTube What Does Mole Represent In Chemistry The term “mole” is defined as the quantity or mass of a substance that contains 6.02 × 10 23 particles (atoms, molecules, or formula units). The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. Here's a look at what a mole is, why we use moles, and how to convert between moles. What Does Mole Represent In Chemistry.
From courses.lumenlearning.com
Formula Mass and the Mole Concept General Chemistry What Does Mole Represent In Chemistry It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. It is defined as the amount of a. Mole in chemistry the mole is an si unit used to. A mole is a unit of measurement that. What Does Mole Represent In Chemistry.
From www.slideserve.com
PPT MOLE RATIOS IN CHEMICAL EQUATIONS PowerPoint Presentation, free What Does Mole Represent In Chemistry It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). The mole is the unit of measurement in the international system of units (si) for amount of substance. The term “mole” is defined as the quantity or mass of a substance that contains 6.02 × 10 23 particles (atoms, molecules, or formula units). Mole,. What Does Mole Represent In Chemistry.
From sciencenotes.org
What Is a Mole In Chemistry? Definition What Does Mole Represent In Chemistry 6.02x10 23 is a unique number, called avogadro’s number. It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). Updated on july 03, 2019. The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. A mole is defined as a chemical unit, defined to be 6.022 x. What Does Mole Represent In Chemistry.
From www.slideserve.com
PPT The Mole PowerPoint Presentation, free download ID5771441 What Does Mole Represent In Chemistry The term “mole” is defined as the quantity or mass of a substance that contains 6.02 × 10 23 particles (atoms, molecules, or formula units). Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. 6.02x10 23 is a unique number, called avogadro’s number. A mole is a unit of measurement. What Does Mole Represent In Chemistry.
From quizlet.com
Chem 08 Reactions & Moles Diagram Quizlet What Does Mole Represent In Chemistry Mole in chemistry the mole is an si unit used to. 6.02x10 23 is a unique number, called avogadro’s number. It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). A mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. The mole is the unit of. What Does Mole Represent In Chemistry.
From www.youtube.com
What is a mole? Definition and examples YouTube What Does Mole Represent In Chemistry A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. The mole is the unit of measurement in the international system of units (si) for amount of substance. Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. Here's a look. What Does Mole Represent In Chemistry.
From www.youtube.com
Mole Concept Class 11 Chemistry YouTube What Does Mole Represent In Chemistry The mole is the unit of measurement in the international system of units (si) for amount of substance. A mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). Mole, in chemistry, a standard scientific unit for. What Does Mole Represent In Chemistry.
From upberi.com
Mole Concept Definition, Formula, Examples, and FAQs (2022) What Does Mole Represent In Chemistry The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. It is defined as the amount of a. The mole is the unit of measurement in the international system of units (si) for amount of substance. Here's a look at what a mole is, why we use moles, and how to convert between. What Does Mole Represent In Chemistry.
From telgurus.co.uk
How to calculate moles in chemistry? Chemistry questionnaire What Does Mole Represent In Chemistry A mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. Updated on july 03, 2019. 6.02x10 23 is a unique number, called avogadro’s number. The mole is the unit of measurement in the. What Does Mole Represent In Chemistry.
From mungfali.com
The Mole Chemistry What Does Mole Represent In Chemistry Updated on july 03, 2019. 6.02x10 23 is a unique number, called avogadro’s number. The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. Here's a look at what a mole is, why we use moles, and how to convert between moles and grams. Mole in chemistry the mole is an si unit. What Does Mole Represent In Chemistry.
From www.slideserve.com
PPT MOLE RATIOS IN CHEMICAL EQUATIONS PowerPoint Presentation, free What Does Mole Represent In Chemistry The mole is the unit of measurement in the international system of units (si) for amount of substance. A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. Here's a look at what a mole is, why we use moles, and how to convert between moles and grams. Updated on. What Does Mole Represent In Chemistry.
From www.templateroller.com
Chemistry Cheat Sheet Mole Conversion Chart Download Printable PDF What Does Mole Represent In Chemistry The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. 6.02x10 23 is a unique number, called avogadro’s number. The term “mole” is defined as the quantity or mass of a substance that contains 6.02 × 10 23 particles (atoms, molecules, or formula units). Mole, in chemistry, a standard scientific unit for measuring. What Does Mole Represent In Chemistry.
From afterskool.edu.sg
'O' Level Chemistry 101 Mole Concept Summary Guide — AfterSkool What Does Mole Represent In Chemistry The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. A mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. 6.02x10 23 is a unique number, called avogadro’s number. It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons).. What Does Mole Represent In Chemistry.
From www.pinterest.com
What does a Mole Mean? Chemistry classroom, Chemistry teacher high What Does Mole Represent In Chemistry 6.02x10 23 is a unique number, called avogadro’s number. It is defined as the amount of a. Updated on july 03, 2019. The mole is the unit of measurement in the international system of units (si) for amount of substance. A mole is defined as a chemical unit, defined to be 6.022 x 10 23 (avogadro's constant) entities. It is. What Does Mole Represent In Chemistry.
From www.youtube.com
What is a Chemistry Mole....Explained! YouTube What Does Mole Represent In Chemistry The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. Updated on july 03, 2019. The mole is the unit of measurement in the international system of units (si) for amount of substance. It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). Here's a look at. What Does Mole Represent In Chemistry.
From learningwohrleef.z14.web.core.windows.net
Avogadro's Number And The Mole What Does Mole Represent In Chemistry The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. Here's a look at what a mole is, why we use moles, and how to convert between moles and grams. The term “mole” is defined as the quantity or mass of a substance that contains 6.02 × 10 23 particles (atoms, molecules, or. What Does Mole Represent In Chemistry.
From www.shutterstock.com
Number Moles Formula Chemistry Stock Vector (Royalty Free) 2140739037 What Does Mole Represent In Chemistry The mole is the unit of measurement in the international system of units (si) for amount of substance. It is defined as the amount of a. It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). 6.02x10 23 is a unique number, called avogadro’s number. Mole in chemistry the mole is an si unit. What Does Mole Represent In Chemistry.
From ar.inspiredpencil.com
Mole Chemistry What Does Mole Represent In Chemistry The term “mole” is defined as the quantity or mass of a substance that contains 6.02 × 10 23 particles (atoms, molecules, or formula units). It is defined as the amount of a. A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. 6.02x10 23 is a unique number, called. What Does Mole Represent In Chemistry.
From periodictable.me
Introduction to the Mole in Chemistry A Brief Explanation What Does Mole Represent In Chemistry Updated on july 03, 2019. The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). It is defined as the amount of a. Mole in chemistry the mole is an si unit used to. The term “mole”. What Does Mole Represent In Chemistry.
From www.youtube.com
Understanding Moles (GCSE Chemistry) YouTube What Does Mole Represent In Chemistry Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction. Updated on july 03, 2019. Here's a look at what a mole is, why we use moles, and how to convert. What Does Mole Represent In Chemistry.
From www.slideserve.com
PPT THE MOLE CONCEPT PowerPoint Presentation, free download ID442205 What Does Mole Represent In Chemistry Updated on july 03, 2019. It is defined that 1 mole of any substance contains 6.02x1023 particles (atoms, molecules, ions, electrons). It is defined as the amount of a. The term “mole” is defined as the quantity or mass of a substance that contains 6.02 × 10 23 particles (atoms, molecules, or formula units). Mole in chemistry the mole is. What Does Mole Represent In Chemistry.
From chemistrymysteries.blogspot.com
Chemistry Mysteries Mole Conversions What Does Mole Represent In Chemistry The mole (n), abbreviated as mol, is a fundamental chemical quantity that characterizes the amount of substance. The term “mole” is defined as the quantity or mass of a substance that contains 6.02 × 10 23 particles (atoms, molecules, or formula units). Mole in chemistry the mole is an si unit used to. A mole is defined as a chemical. What Does Mole Represent In Chemistry.