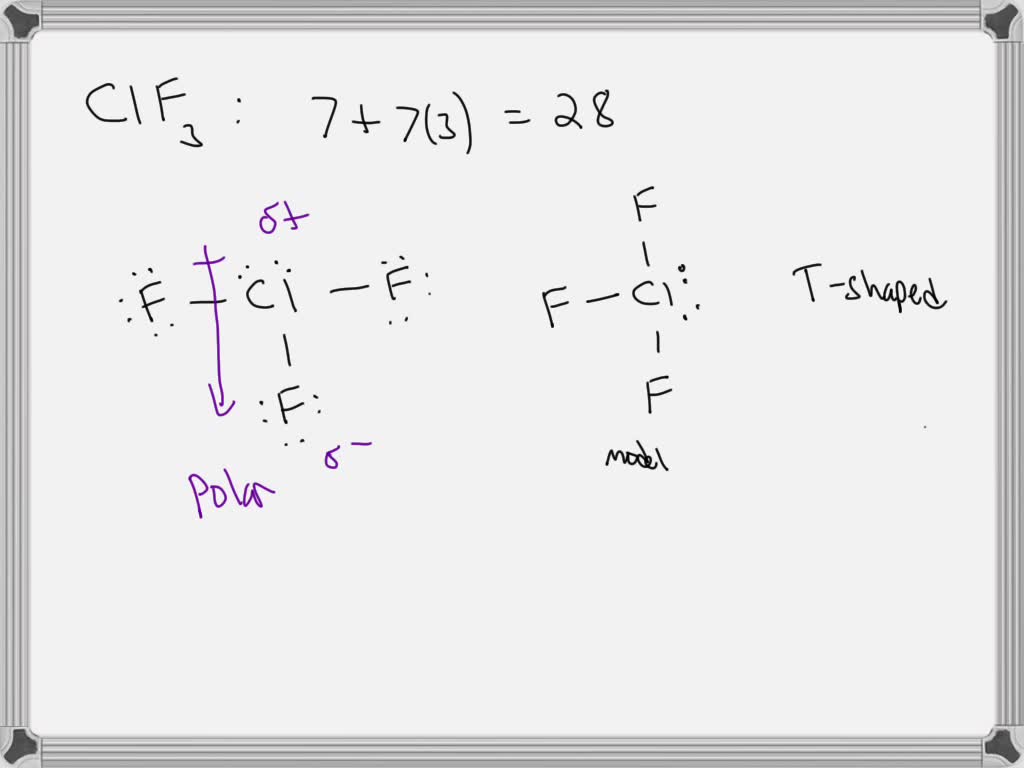Chlorine Trifluoride Vsepr Theory . Valence shell electron pair repulsion theory is a simple. vsepr calculation for chlorine trifluoride, cl f 3. Refer to the previous section for a discussion on the isomer possibilities for this. The two lone pairs take equatorial positions because they demand more space than the bonds. the standard application of vsepr theory to this molecule is as follows: Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). there is an abundance of experimental evidence to that effect—from their physical properties to their chemical. A closer look at chlorine trifluoride, clf3. clf3 chlorine trifluoride. To use the vsepr model to predict molecular geometries. To predict whether a molecule has a dipole moment.
from www.numerade.com
the standard application of vsepr theory to this molecule is as follows: The two lone pairs take equatorial positions because they demand more space than the bonds. Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). clf3 chlorine trifluoride. vsepr calculation for chlorine trifluoride, cl f 3. Refer to the previous section for a discussion on the isomer possibilities for this. there is an abundance of experimental evidence to that effect—from their physical properties to their chemical. To use the vsepr model to predict molecular geometries. Valence shell electron pair repulsion theory is a simple. A closer look at chlorine trifluoride, clf3.
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons
Chlorine Trifluoride Vsepr Theory Refer to the previous section for a discussion on the isomer possibilities for this. vsepr calculation for chlorine trifluoride, cl f 3. To use the vsepr model to predict molecular geometries. To predict whether a molecule has a dipole moment. A closer look at chlorine trifluoride, clf3. there is an abundance of experimental evidence to that effect—from their physical properties to their chemical. clf3 chlorine trifluoride. Refer to the previous section for a discussion on the isomer possibilities for this. Valence shell electron pair repulsion theory is a simple. the standard application of vsepr theory to this molecule is as follows: The two lone pairs take equatorial positions because they demand more space than the bonds. Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs).
From felica8sy-images.blogspot.com
Fcl3 Lewis Structure / Vsepr Clf3 Chlorine Trifluoride / Give each of Chlorine Trifluoride Vsepr Theory To use the vsepr model to predict molecular geometries. Refer to the previous section for a discussion on the isomer possibilities for this. clf3 chlorine trifluoride. vsepr calculation for chlorine trifluoride, cl f 3. there is an abundance of experimental evidence to that effect—from their physical properties to their chemical. Valence shell electron pair repulsion theory is. Chlorine Trifluoride Vsepr Theory.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Chlorine Trifluoride Vsepr Theory Refer to the previous section for a discussion on the isomer possibilities for this. the standard application of vsepr theory to this molecule is as follows: A closer look at chlorine trifluoride, clf3. vsepr calculation for chlorine trifluoride, cl f 3. To use the vsepr model to predict molecular geometries. clf3 chlorine trifluoride. To predict whether a. Chlorine Trifluoride Vsepr Theory.
From ar.inspiredpencil.com
Lewis Structure For Clf3 Chlorine Trifluoride Vsepr Theory To predict whether a molecule has a dipole moment. A closer look at chlorine trifluoride, clf3. clf3 chlorine trifluoride. the standard application of vsepr theory to this molecule is as follows: Refer to the previous section for a discussion on the isomer possibilities for this. vsepr calculation for chlorine trifluoride, cl f 3. Valence shell electron pair. Chlorine Trifluoride Vsepr Theory.
From galeria-szablonow.blogspot.com
Chlorine Pentafluoride Vsepr / Physical and chemical properties Chlorine Trifluoride Vsepr Theory To predict whether a molecule has a dipole moment. A closer look at chlorine trifluoride, clf3. Refer to the previous section for a discussion on the isomer possibilities for this. vsepr calculation for chlorine trifluoride, cl f 3. To use the vsepr model to predict molecular geometries. Chlorine trifluoride has 5 regions of electron density around the central chlorine. Chlorine Trifluoride Vsepr Theory.
From www.anyrgb.com
Binary Phase, Xenon difluoride, Xenon tetrafluoride, chlorine Chlorine Trifluoride Vsepr Theory there is an abundance of experimental evidence to that effect—from their physical properties to their chemical. the standard application of vsepr theory to this molecule is as follows: To use the vsepr model to predict molecular geometries. A closer look at chlorine trifluoride, clf3. Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3. Chlorine Trifluoride Vsepr Theory.
From www.slideserve.com
PPT VSEPR. PowerPoint Presentation ID214953 Chlorine Trifluoride Vsepr Theory Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). Refer to the previous section for a discussion on the isomer possibilities for this. The two lone pairs take equatorial positions because they demand more space than the bonds. vsepr calculation for chlorine trifluoride, cl f 3. the standard. Chlorine Trifluoride Vsepr Theory.
From www.studocu.com
Chlorine Trifluoride M.O. Diagram CHEM12A Studocu Chlorine Trifluoride Vsepr Theory Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). Refer to the previous section for a discussion on the isomer possibilities for this. The two lone pairs take equatorial positions because they demand more space than the bonds. A closer look at chlorine trifluoride, clf3. clf3 chlorine trifluoride. . Chlorine Trifluoride Vsepr Theory.
From jsmithmoore.com
Vsepr theory chart Chlorine Trifluoride Vsepr Theory vsepr calculation for chlorine trifluoride, cl f 3. The two lone pairs take equatorial positions because they demand more space than the bonds. clf3 chlorine trifluoride. To use the vsepr model to predict molecular geometries. the standard application of vsepr theory to this molecule is as follows: Chlorine trifluoride has 5 regions of electron density around the. Chlorine Trifluoride Vsepr Theory.
From iccontrols.com
Chlorine Theory and Measurements IC Controls Chlorine Trifluoride Vsepr Theory To predict whether a molecule has a dipole moment. The two lone pairs take equatorial positions because they demand more space than the bonds. Valence shell electron pair repulsion theory is a simple. there is an abundance of experimental evidence to that effect—from their physical properties to their chemical. clf3 chlorine trifluoride. vsepr calculation for chlorine trifluoride,. Chlorine Trifluoride Vsepr Theory.
From interestingengineering.com
Chlorine Trifluoride The Compound That Can Even Set Fire to Glass Chlorine Trifluoride Vsepr Theory Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). To use the vsepr model to predict molecular geometries. Refer to the previous section for a discussion on the isomer possibilities for this. To predict whether a molecule has a dipole moment. there is an abundance of experimental evidence to. Chlorine Trifluoride Vsepr Theory.
From www.youtube.com
VSEPR Molecular Geometry TShaped Chlorine Trifluoride Chlorine Trifluoride Vsepr Theory clf3 chlorine trifluoride. To predict whether a molecule has a dipole moment. The two lone pairs take equatorial positions because they demand more space than the bonds. To use the vsepr model to predict molecular geometries. vsepr calculation for chlorine trifluoride, cl f 3. there is an abundance of experimental evidence to that effect—from their physical properties. Chlorine Trifluoride Vsepr Theory.
From www.numerade.com
SOLVED Gaseous chlorine trifluoride is a compound used in the Chlorine Trifluoride Vsepr Theory Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). To predict whether a molecule has a dipole moment. the standard application of vsepr theory to this molecule is as follows: A closer look at chlorine trifluoride, clf3. The two lone pairs take equatorial positions because they demand more space. Chlorine Trifluoride Vsepr Theory.
From www.youtube.com
IB ChemistryVSEPRChlorine Trifluoride YouTube Chlorine Trifluoride Vsepr Theory To use the vsepr model to predict molecular geometries. the standard application of vsepr theory to this molecule is as follows: there is an abundance of experimental evidence to that effect—from their physical properties to their chemical. A closer look at chlorine trifluoride, clf3. Valence shell electron pair repulsion theory is a simple. Refer to the previous section. Chlorine Trifluoride Vsepr Theory.
From democracyunlimited.web.fc2.com
molecular shape of sf4 Chlorine Trifluoride Vsepr Theory Valence shell electron pair repulsion theory is a simple. clf3 chlorine trifluoride. The two lone pairs take equatorial positions because they demand more space than the bonds. Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). the standard application of vsepr theory to this molecule is as follows:. Chlorine Trifluoride Vsepr Theory.
From chem.libretexts.org
10.2 The VSEPR Model Chemistry LibreTexts Chlorine Trifluoride Vsepr Theory vsepr calculation for chlorine trifluoride, cl f 3. there is an abundance of experimental evidence to that effect—from their physical properties to their chemical. Valence shell electron pair repulsion theory is a simple. To use the vsepr model to predict molecular geometries. A closer look at chlorine trifluoride, clf3. the standard application of vsepr theory to this. Chlorine Trifluoride Vsepr Theory.
From www.youtube.com
Molecular Structure of Chlorine Trifluoride Using VSEPR Theory Nature Chlorine Trifluoride Vsepr Theory vsepr calculation for chlorine trifluoride, cl f 3. Refer to the previous section for a discussion on the isomer possibilities for this. To predict whether a molecule has a dipole moment. Valence shell electron pair repulsion theory is a simple. there is an abundance of experimental evidence to that effect—from their physical properties to their chemical. the. Chlorine Trifluoride Vsepr Theory.
From www.ch.imperial.ac.uk
A wider look at chlorine trifluoride crystal structures and data Chlorine Trifluoride Vsepr Theory The two lone pairs take equatorial positions because they demand more space than the bonds. Refer to the previous section for a discussion on the isomer possibilities for this. To use the vsepr model to predict molecular geometries. A closer look at chlorine trifluoride, clf3. Valence shell electron pair repulsion theory is a simple. clf3 chlorine trifluoride. there. Chlorine Trifluoride Vsepr Theory.
From www.youtube.com
VSEPR Theory (Structure) Chemical Bonding & Molecular Structure Chlorine Trifluoride Vsepr Theory vsepr calculation for chlorine trifluoride, cl f 3. there is an abundance of experimental evidence to that effect—from their physical properties to their chemical. the standard application of vsepr theory to this molecule is as follows: To use the vsepr model to predict molecular geometries. Refer to the previous section for a discussion on the isomer possibilities. Chlorine Trifluoride Vsepr Theory.
From www.youtube.com
ClF3 Hybridization (Chlorine Trifluoride) YouTube Chlorine Trifluoride Vsepr Theory A closer look at chlorine trifluoride, clf3. Valence shell electron pair repulsion theory is a simple. To predict whether a molecule has a dipole moment. Refer to the previous section for a discussion on the isomer possibilities for this. The two lone pairs take equatorial positions because they demand more space than the bonds. Chlorine trifluoride has 5 regions of. Chlorine Trifluoride Vsepr Theory.
From www.academia.edu
(PDF) VSEPR Theory A closer look at chlorine trifluoride, ClF3 Henry Chlorine Trifluoride Vsepr Theory Refer to the previous section for a discussion on the isomer possibilities for this. Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). vsepr calculation for chlorine trifluoride, cl f 3. To predict whether a molecule has a dipole moment. To use the vsepr model to predict molecular geometries.. Chlorine Trifluoride Vsepr Theory.
From wisc.pb.unizin.org
Day 11 Resonance Structures, VSEPR Theory Chemistry 109 Chlorine Trifluoride Vsepr Theory there is an abundance of experimental evidence to that effect—from their physical properties to their chemical. A closer look at chlorine trifluoride, clf3. clf3 chlorine trifluoride. vsepr calculation for chlorine trifluoride, cl f 3. Refer to the previous section for a discussion on the isomer possibilities for this. Chlorine trifluoride has 5 regions of electron density around. Chlorine Trifluoride Vsepr Theory.
From www.vrogue.co
10 2 Vsepr Theory The Five Basic Shapes Chemistry Lib vrogue.co Chlorine Trifluoride Vsepr Theory Refer to the previous section for a discussion on the isomer possibilities for this. Valence shell electron pair repulsion theory is a simple. A closer look at chlorine trifluoride, clf3. The two lone pairs take equatorial positions because they demand more space than the bonds. To predict whether a molecule has a dipole moment. To use the vsepr model to. Chlorine Trifluoride Vsepr Theory.
From study.com
VSEPR Theory Chart & Model Lesson Chlorine Trifluoride Vsepr Theory Valence shell electron pair repulsion theory is a simple. vsepr calculation for chlorine trifluoride, cl f 3. The two lone pairs take equatorial positions because they demand more space than the bonds. To use the vsepr model to predict molecular geometries. A closer look at chlorine trifluoride, clf3. Chlorine trifluoride has 5 regions of electron density around the central. Chlorine Trifluoride Vsepr Theory.
From www.youtube.com
ClF3 Lewis Structure (Chlorine Trifluoride) YouTube Chlorine Trifluoride Vsepr Theory Refer to the previous section for a discussion on the isomer possibilities for this. clf3 chlorine trifluoride. The two lone pairs take equatorial positions because they demand more space than the bonds. the standard application of vsepr theory to this molecule is as follows: there is an abundance of experimental evidence to that effect—from their physical properties. Chlorine Trifluoride Vsepr Theory.
From www.youtube.com
Is ClF3 Polar or Nonpolar (Chlorine trifluoride) YouTube Chlorine Trifluoride Vsepr Theory Refer to the previous section for a discussion on the isomer possibilities for this. the standard application of vsepr theory to this molecule is as follows: vsepr calculation for chlorine trifluoride, cl f 3. Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). To use the vsepr model. Chlorine Trifluoride Vsepr Theory.
From www.youtube.com
ClF3 Chlorine Trifluoride Shape Hybridisation VSEPR Problem Chlorine Trifluoride Vsepr Theory there is an abundance of experimental evidence to that effect—from their physical properties to their chemical. To use the vsepr model to predict molecular geometries. the standard application of vsepr theory to this molecule is as follows: To predict whether a molecule has a dipole moment. The two lone pairs take equatorial positions because they demand more space. Chlorine Trifluoride Vsepr Theory.
From iccontrols.com
Chlorine Theory and Measurements IC Controls Chlorine Trifluoride Vsepr Theory Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). To predict whether a molecule has a dipole moment. Valence shell electron pair repulsion theory is a simple. vsepr calculation for chlorine trifluoride, cl f 3. The two lone pairs take equatorial positions because they demand more space than the. Chlorine Trifluoride Vsepr Theory.
From www.numerade.com
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons Chlorine Trifluoride Vsepr Theory A closer look at chlorine trifluoride, clf3. To predict whether a molecule has a dipole moment. Valence shell electron pair repulsion theory is a simple. Refer to the previous section for a discussion on the isomer possibilities for this. To use the vsepr model to predict molecular geometries. Chlorine trifluoride has 5 regions of electron density around the central chlorine. Chlorine Trifluoride Vsepr Theory.
From imgbin.com
Chlorine Pentafluoride Antimony Pentafluoride Chlorine Trifluoride Chlorine Trifluoride Vsepr Theory vsepr calculation for chlorine trifluoride, cl f 3. To use the vsepr model to predict molecular geometries. Refer to the previous section for a discussion on the isomer possibilities for this. there is an abundance of experimental evidence to that effect—from their physical properties to their chemical. clf3 chlorine trifluoride. Valence shell electron pair repulsion theory is. Chlorine Trifluoride Vsepr Theory.
From www.vedantu.com
The shape of ClF_3 according to VSEPR model isa.) Planar triangleb Chlorine Trifluoride Vsepr Theory clf3 chlorine trifluoride. To predict whether a molecule has a dipole moment. A closer look at chlorine trifluoride, clf3. there is an abundance of experimental evidence to that effect—from their physical properties to their chemical. the standard application of vsepr theory to this molecule is as follows: To use the vsepr model to predict molecular geometries. Chlorine. Chlorine Trifluoride Vsepr Theory.
From www.numerade.com
SOLVEDUse the VSEPR model to help you describe the bonding in the Chlorine Trifluoride Vsepr Theory Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). A closer look at chlorine trifluoride, clf3. the standard application of vsepr theory to this molecule is as follows: clf3 chlorine trifluoride. Valence shell electron pair repulsion theory is a simple. there is an abundance of experimental evidence. Chlorine Trifluoride Vsepr Theory.
From www.ch.imperial.ac.uk
VSEPR Theory A closer look at bromine trifluoride, BrF3. Henry Rzepa Chlorine Trifluoride Vsepr Theory Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). To use the vsepr model to predict molecular geometries. the standard application of vsepr theory to this molecule is as follows: clf3 chlorine trifluoride. To predict whether a molecule has a dipole moment. vsepr calculation for chlorine trifluoride,. Chlorine Trifluoride Vsepr Theory.
From galeria-szablonow.blogspot.com
Chlorine Pentafluoride Vsepr / Physical and chemical properties Chlorine Trifluoride Vsepr Theory Refer to the previous section for a discussion on the isomer possibilities for this. To predict whether a molecule has a dipole moment. To use the vsepr model to predict molecular geometries. A closer look at chlorine trifluoride, clf3. the standard application of vsepr theory to this molecule is as follows: there is an abundance of experimental evidence. Chlorine Trifluoride Vsepr Theory.
From ajorpng.blogspot.com
Chlorine Pentafluoride Vsepr / The gas chlorine pentafluoride (clf5 Chlorine Trifluoride Vsepr Theory Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). To use the vsepr model to predict molecular geometries. The two lone pairs take equatorial positions because they demand more space than the bonds. Refer to the previous section for a discussion on the isomer possibilities for this. clf3 chlorine. Chlorine Trifluoride Vsepr Theory.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Chlorine Trifluoride Vsepr Theory Chlorine trifluoride has 5 regions of electron density around the central chlorine atom (3 bonds and 2 lone pairs). clf3 chlorine trifluoride. the standard application of vsepr theory to this molecule is as follows: To predict whether a molecule has a dipole moment. Valence shell electron pair repulsion theory is a simple. there is an abundance of. Chlorine Trifluoride Vsepr Theory.