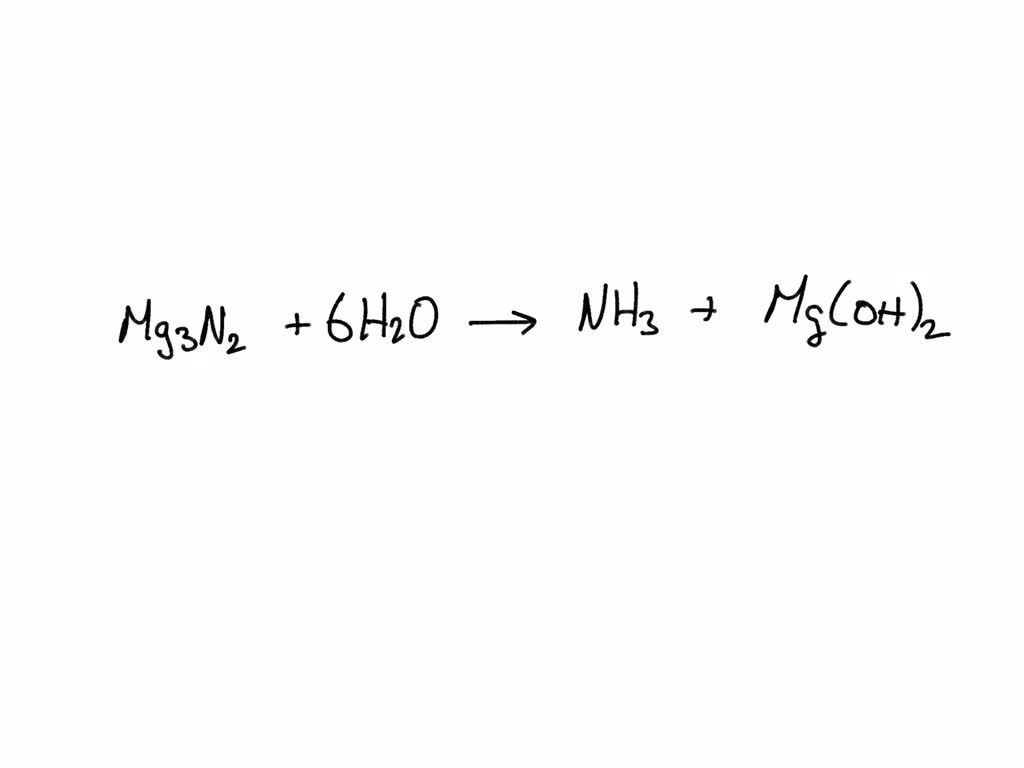Magnesium In Water Chemistry . the reaction of magnesium metal and water comes up quite frequently in chemistry. part of ncssm core collection: Reaction mechanisms, environmental impact and health effects. Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. In this video we'll look at the. when finely powdered, magnesium reacts with water to produce hydrogen gas: when magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. \[ mg_{(s)} + h_2o_{(g)} \rightarrow. This video shows the physical properties of mg metal and its reaction with water. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas.
from www.numerade.com
Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. part of ncssm core collection: \[ mg_{(s)} + h_2o_{(g)} \rightarrow. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. when finely powdered, magnesium reacts with water to produce hydrogen gas: the reaction of magnesium metal and water comes up quite frequently in chemistry. In this video we'll look at the. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. when magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. This video shows the physical properties of mg metal and its reaction with water.
SOLVED Write the balanced equation for Magnesium nitride + water > magnesium hydroxide
Magnesium In Water Chemistry This video shows the physical properties of mg metal and its reaction with water. when finely powdered, magnesium reacts with water to produce hydrogen gas: the reaction of magnesium metal and water comes up quite frequently in chemistry. In this video we'll look at the. Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. This video shows the physical properties of mg metal and its reaction with water. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. \[ mg_{(s)} + h_2o_{(g)} \rightarrow. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. part of ncssm core collection: when magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. Reaction mechanisms, environmental impact and health effects.
From drdavisinfinitehealth.com
Magnesium Water StepbyStep Dr. William Davis Magnesium In Water Chemistry Reaction mechanisms, environmental impact and health effects. when finely powdered, magnesium reacts with water to produce hydrogen gas: Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. In this video we'll look at the. \[ mg_{(s)} + h_2o_{(g)} \rightarrow. This video shows the physical. Magnesium In Water Chemistry.
From fphoto.photoshelter.com
science chemistry redox reaction magnesium hydrochloric acid Fundamental Photographs The Art Magnesium In Water Chemistry Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. Reaction mechanisms, environmental impact and health effects. \[ mg_{(s)} + h_2o_{(g)} \rightarrow. In this video we'll look at the. part. Magnesium In Water Chemistry.
From www.britannica.com
magnesium Description, Properties, & Compounds Britannica Magnesium In Water Chemistry Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. the reaction of magnesium metal and water comes up quite frequently in chemistry. Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. This video shows the physical properties of. Magnesium In Water Chemistry.
From mavink.com
Magnesium Reacts With Hydrochloric Acid Magnesium In Water Chemistry \[ mg_{(s)} + h_2o_{(g)} \rightarrow. Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. Reaction mechanisms, environmental impact and health effects. This video shows the physical properties of mg metal and its reaction with water. Magnesium burns in steam to produce white. Magnesium In Water Chemistry.
From www.sciencephoto.com
Magnesium reacting with water Stock Image A500/0369 Science Photo Library Magnesium In Water Chemistry \[ mg_{(s)} + h_2o_{(g)} \rightarrow. In this video we'll look at the. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. when magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. the reaction of magnesium metal and water comes up quite frequently in chemistry. when finely powdered,. Magnesium In Water Chemistry.
From www.youtube.com
Equation for Mg(NO3)2 + H2O (Magnesium nitrate + Water) YouTube Magnesium In Water Chemistry In this video we'll look at the. Reaction mechanisms, environmental impact and health effects. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. when finely powdered, magnesium reacts with water to produce hydrogen gas: When exposed to steam, magnesium changes from magnesium to magnesium oxide and. This video shows the physical properties of mg metal and. Magnesium In Water Chemistry.
From fphoto.photoshelter.com
science chemistry redox reaction magnesium hydrochloric acid Fundamental Photographs The Art Magnesium In Water Chemistry when finely powdered, magnesium reacts with water to produce hydrogen gas: When exposed to steam, magnesium changes from magnesium to magnesium oxide and. This video shows the physical properties of mg metal and its reaction with water. Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. \[ mg_{(s)} + h_2o_{(g)} \rightarrow. In this. Magnesium In Water Chemistry.
From studyvillainies.z13.web.core.windows.net
What Is Magnesium's Density Magnesium In Water Chemistry This video shows the physical properties of mg metal and its reaction with water. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. \[ mg_{(s)} + h_2o_{(g)} \rightarrow. when finely powdered, magnesium reacts with water to produce hydrogen gas: Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Reaction mechanisms, environmental impact and. Magnesium In Water Chemistry.
From www.youtube.com
Reaction between Magnesium and Water (Mg + H2O) YouTube Magnesium In Water Chemistry part of ncssm core collection: Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. This video shows the physical properties of mg metal and its reaction with water. Reaction mechanisms, environmental impact and health effects. \[ mg_{(s)} + h_2o_{(g)} \rightarrow. when magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single. Magnesium In Water Chemistry.
From www.researchgate.net
2) gives the solubility of magnesium in water at 25°C as a function of... Download Scientific Magnesium In Water Chemistry When exposed to steam, magnesium changes from magnesium to magnesium oxide and. \[ mg_{(s)} + h_2o_{(g)} \rightarrow. when magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. part of ncssm core collection: Mg(s) + 2 h 2 o(g) →. Magnesium In Water Chemistry.
From www.youtube.com
Mg(OH)2+HNO3=Mg(NO3)2+H2O Balanced EquationMagnesium Hydroxide+Nitric acid=Magnesium Nitrate Magnesium In Water Chemistry when finely powdered, magnesium reacts with water to produce hydrogen gas: Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. part of ncssm core collection: the reaction of magnesium metal and water comes up quite frequently in chemistry. when magnesium reacts with liquid water, it makes mg (oh)2 in a. Magnesium In Water Chemistry.
From ar.inspiredpencil.com
Magnesium And Water Reaction Magnesium In Water Chemistry This video shows the physical properties of mg metal and its reaction with water. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. the reaction of magnesium metal and water comes up quite frequently in chemistry. when magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. Magnesium burns. Magnesium In Water Chemistry.
From pixels.com
Reactivity Of Magnesium In Water Photograph by Andrew Lambert Photography/science Photo Library Magnesium In Water Chemistry the reaction of magnesium metal and water comes up quite frequently in chemistry. This video shows the physical properties of mg metal and its reaction with water. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. \[ mg_{(s)} + h_2o_{(g)} \rightarrow.. Magnesium In Water Chemistry.
From blog.thepipingmart.com
The Chemical Properties of Magnesium Magnesium In Water Chemistry This video shows the physical properties of mg metal and its reaction with water. when magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. Reaction mechanisms, environmental impact and health effects. when finely powdered, magnesium reacts with water to produce hydrogen gas: Mg(s) + 2 h 2 o(g) → mg(oh) 2. Magnesium In Water Chemistry.
From ar.inspiredpencil.com
Magnesium And Water Magnesium In Water Chemistry Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. part of ncssm core collection: In this video we'll look at the. the reaction of magnesium metal and water comes up quite frequently in chemistry. Reaction mechanisms, environmental impact and health effects. This video shows the physical properties of mg metal and its. Magnesium In Water Chemistry.
From ar.inspiredpencil.com
Magnesium And Water Magnesium In Water Chemistry Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. In this video we'll look at the. part of ncssm core collection: when finely powdered, magnesium reacts with water to produce hydrogen gas: Reaction mechanisms, environmental impact and health effects. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. Mg(s) + 2 h. Magnesium In Water Chemistry.
From www.youtube.com
Net Ionic Equation for Mg + HCl (Magnesium + Hydrochloric acid) YouTube Magnesium In Water Chemistry This video shows the physical properties of mg metal and its reaction with water. Reaction mechanisms, environmental impact and health effects. Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. In this video we'll look at the. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. \[ mg_{(s)} + h_2o_{(g)} \rightarrow.. Magnesium In Water Chemistry.
From www.youtube.com
Why does Magnesium react with Water? YouTube Magnesium In Water Chemistry This video shows the physical properties of mg metal and its reaction with water. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. \[ mg_{(s)} + h_2o_{(g)} \rightarrow. the reaction of magnesium metal and water comes up quite frequently in chemistry. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. In this video. Magnesium In Water Chemistry.
From www.sciencephoto.com
Magnesium reacts with water Stock Image C028/0749 Science Photo Library Magnesium In Water Chemistry When exposed to steam, magnesium changes from magnesium to magnesium oxide and. This video shows the physical properties of mg metal and its reaction with water. when finely powdered, magnesium reacts with water to produce hydrogen gas: Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. Magnesium burns in steam to produce white. Magnesium In Water Chemistry.
From www.tessshebaylo.com
Magnesium And Hot Water Equation Tessshebaylo Magnesium In Water Chemistry \[ mg_{(s)} + h_2o_{(g)} \rightarrow. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Reaction mechanisms, environmental impact and health effects. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. when magnesium reacts with liquid water, it makes. Magnesium In Water Chemistry.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Magnesium In Water Chemistry When exposed to steam, magnesium changes from magnesium to magnesium oxide and. Reaction mechanisms, environmental impact and health effects. This video shows the physical properties of mg metal and its reaction with water. part of ncssm core collection: \[ mg_{(s)} + h_2o_{(g)} \rightarrow. when magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single. Magnesium In Water Chemistry.
From www.numerade.com
SOLVED Write the balanced equation for Magnesium nitride + water > magnesium hydroxide Magnesium In Water Chemistry Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. when magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. part of ncssm core collection: Reaction mechanisms, environmental impact and health effects. \[ mg_{(s)}. Magnesium In Water Chemistry.
From www.chemistryworld.com
Magnesium Podcast Chemistry World Magnesium In Water Chemistry Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. when magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. when finely powdered, magnesium reacts with water to produce hydrogen gas: In this video we'll look at the. the reaction of magnesium metal and water comes up quite. Magnesium In Water Chemistry.
From www.youtube.com
How to Balance Mg + H2O = MgO + H2 Magnesium + Water (steam) YouTube Magnesium In Water Chemistry the reaction of magnesium metal and water comes up quite frequently in chemistry. when finely powdered, magnesium reacts with water to produce hydrogen gas: Reaction mechanisms, environmental impact and health effects. part of ncssm core collection: In this video we'll look at the. \[ mg_{(s)} + h_2o_{(g)} \rightarrow. When exposed to steam, magnesium changes from magnesium to. Magnesium In Water Chemistry.
From honorchemistry.weebly.com
Magnesium Sulfate Experiment! Magnesium In Water Chemistry part of ncssm core collection: Reaction mechanisms, environmental impact and health effects. \[ mg_{(s)} + h_2o_{(g)} \rightarrow. Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. In this video we'll look at the. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. the reaction of magnesium metal and water. Magnesium In Water Chemistry.
From www.examples.com
Magnesium (Mg) Definition, Preparation, Properties, Uses, Compounds, Reactivity Magnesium In Water Chemistry In this video we'll look at the. This video shows the physical properties of mg metal and its reaction with water. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. when finely powdered, magnesium reacts with water to produce hydrogen gas: the reaction of magnesium metal and water comes up quite frequently in chemistry. . Magnesium In Water Chemistry.
From drdavisinfinitehealth.com
Magnesium Water StepbyStep Dr. William Davis Magnesium In Water Chemistry When exposed to steam, magnesium changes from magnesium to magnesium oxide and. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. when magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. when finely powdered, magnesium reacts with water to produce hydrogen gas: Mg(s) + 2 h 2 o(g). Magnesium In Water Chemistry.
From ar.inspiredpencil.com
Magnesium And Water Reaction Magnesium In Water Chemistry In this video we'll look at the. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. the reaction of magnesium metal and water comes up quite frequently in chemistry. Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. when finely powdered, magnesium reacts with water to produce hydrogen gas:. Magnesium In Water Chemistry.
From www.sciencephoto.com
Magnesium oxide reacts with water Stock Image C030/8190 Science Photo Library Magnesium In Water Chemistry When exposed to steam, magnesium changes from magnesium to magnesium oxide and. Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. when magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. In this video. Magnesium In Water Chemistry.
From www.elektramagnesium.com.au
Ionic Magnesium Chloride Hexahydrate or Chelated Magnesium? Elektra Magnesium Magnesium In Water Chemistry Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. In this video we'll look at the. This video shows the physical properties of mg metal and its reaction with water. \[ mg_{(s)} + h_2o_{(g)} \rightarrow. when finely powdered, magnesium reacts with water to produce. Magnesium In Water Chemistry.
From ar.inspiredpencil.com
Magnesium And Water Magnesium In Water Chemistry When exposed to steam, magnesium changes from magnesium to magnesium oxide and. This video shows the physical properties of mg metal and its reaction with water. when finely powdered, magnesium reacts with water to produce hydrogen gas: when magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. \[ mg_{(s)} + h_2o_{(g)}. Magnesium In Water Chemistry.
From www.alamy.com
Labelled diagram of magnesium reacting with steam. Vector diagram for educational use Stock Magnesium In Water Chemistry Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. This video shows the physical properties of mg metal and its reaction with water. \[ mg_{(s)} + h_2o_{(g)} \rightarrow. the reaction of magnesium metal and water comes up quite frequently in chemistry. when magnesium reacts with liquid water, it makes mg (oh)2 in. Magnesium In Water Chemistry.
From fphoto.photoshelter.com
science chemistry redox reaction magnesium hydrochloric acid Fundamental Photographs The Art Magnesium In Water Chemistry part of ncssm core collection: \[ mg_{(s)} + h_2o_{(g)} \rightarrow. when magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. In this video we'll look at the. when finely powdered, magnesium reacts with water to. Magnesium In Water Chemistry.
From www.slideshare.net
Chemical Reactions Magnesium In Water Chemistry This video shows the physical properties of mg metal and its reaction with water. the reaction of magnesium metal and water comes up quite frequently in chemistry. Mg(s) + 2 h 2 o(g) → mg(oh) 2 (aq) + h 2 (g) +. part of ncssm core collection: when finely powdered, magnesium reacts with water to produce hydrogen. Magnesium In Water Chemistry.
From media.ed.science.psu.edu
Solubility of ionic solids as a function of temperature Magnesium In Water Chemistry When exposed to steam, magnesium changes from magnesium to magnesium oxide and. This video shows the physical properties of mg metal and its reaction with water. the reaction of magnesium metal and water comes up quite frequently in chemistry. when finely powdered, magnesium reacts with water to produce hydrogen gas: Magnesium burns in steam to produce white magnesium. Magnesium In Water Chemistry.