What Would Be The Boiling Point Of Pure Water . the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. Usually, you'll find that these values are. for instance, at a standard atmospheric pressure, pure water has a fixed boiling point. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere. The normal boiling point is the temperature at which the vapour pressure is equal. what’s the boiling point of water? the boiling point of a liquid varies according to the applied pressure; The boiling point of water is 99.97 °c, or 211.95 °f , under standard pressure at sea level. the boiling point is the temperature at which a liquid substance changes to its gaseous phase. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. However, if impurities such as sodium chloride or the common. The standard boiling point of water is 99.61 °c at 1 bar of pressure.
from schematicfritolay40sdrtk.z13.web.core.windows.net
if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere. the boiling point of a liquid varies according to the applied pressure; the boiling point is the temperature at which a liquid substance changes to its gaseous phase. The normal boiling point is the temperature at which the vapour pressure is equal. what’s the boiling point of water? The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. The boiling point of water is 99.97 °c, or 211.95 °f , under standard pressure at sea level. the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. Usually, you'll find that these values are. However, if impurities such as sodium chloride or the common.
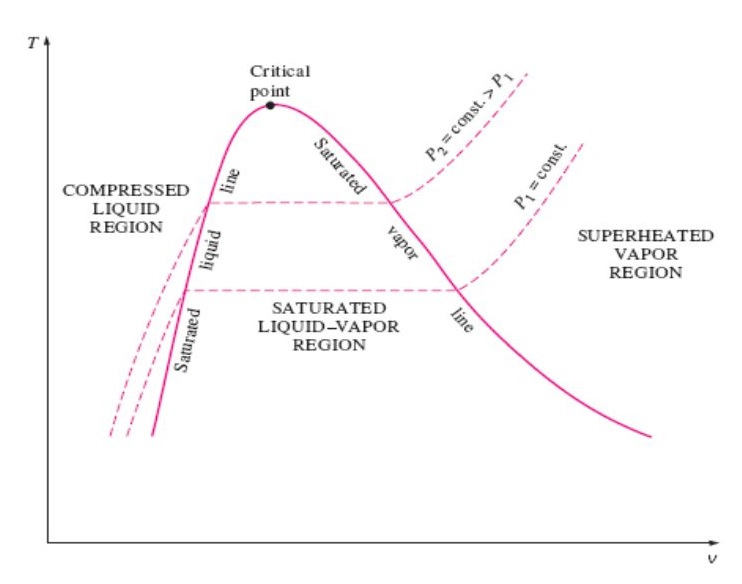
T V Diagram Of Water
What Would Be The Boiling Point Of Pure Water The standard boiling point of water is 99.61 °c at 1 bar of pressure. The boiling point of water is 99.97 °c, or 211.95 °f , under standard pressure at sea level. the boiling point is the temperature at which a liquid substance changes to its gaseous phase. the boiling point of a liquid varies according to the applied pressure; The normal boiling point is the temperature at which the vapour pressure is equal. for instance, at a standard atmospheric pressure, pure water has a fixed boiling point. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. what’s the boiling point of water? the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The standard boiling point of water is 99.61 °c at 1 bar of pressure. However, if impurities such as sodium chloride or the common. Usually, you'll find that these values are. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere.
From www.youtube.com
Does Salt Water Boil Faster? Experiment YouTube What Would Be The Boiling Point Of Pure Water if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere. what’s the boiling point of water? The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. The standard. What Would Be The Boiling Point Of Pure Water.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Would Be The Boiling Point Of Pure Water The boiling point of water is 99.97 °c, or 211.95 °f , under standard pressure at sea level. the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. the boiling point is the temperature at which a liquid substance changes to its gaseous phase. The standard boiling. What Would Be The Boiling Point Of Pure Water.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry What Would Be The Boiling Point Of Pure Water what’s the boiling point of water? However, if impurities such as sodium chloride or the common. The normal boiling point is the temperature at which the vapour pressure is equal. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere. for. What Would Be The Boiling Point Of Pure Water.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii What Would Be The Boiling Point Of Pure Water The boiling point of water is 99.97 °c, or 211.95 °f , under standard pressure at sea level. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. The normal boiling point is the temperature at which the vapour pressure is equal. However, if impurities such. What Would Be The Boiling Point Of Pure Water.
From www.thespruceeats.com
The Boiling Point of Water at Various Altitudes What Would Be The Boiling Point Of Pure Water if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere. the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. Usually, you'll find that these values are. The normal boiling point. What Would Be The Boiling Point Of Pure Water.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Would Be The Boiling Point Of Pure Water for instance, at a standard atmospheric pressure, pure water has a fixed boiling point. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. what’s the boiling point of water? the normal boiling point or the atmospheric boiling point is the boiling point. What Would Be The Boiling Point Of Pure Water.
From www.researchgate.net
Melting and boiling points of select salt compounds Download Table What Would Be The Boiling Point Of Pure Water However, if impurities such as sodium chloride or the common. the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. the boiling point of a liquid varies according to the applied pressure; if you want a quick and simple answer, you can say that the boiling. What Would Be The Boiling Point Of Pure Water.
From vandewaterbooks.com
What Temperature Does Water Boil At? Boiling Point & Elevation Vande What Would Be The Boiling Point Of Pure Water The normal boiling point is the temperature at which the vapour pressure is equal. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level.. What Would Be The Boiling Point Of Pure Water.
From www.youtube.com
Freezing and Boiling Point Graph YouTube What Would Be The Boiling Point Of Pure Water if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere. The boiling point of water is 99.97 °c, or 211.95 °f , under standard pressure at sea level. what’s the boiling point of water? The normal boiling point is the temperature at. What Would Be The Boiling Point Of Pure Water.
From chem.libretexts.org
10.4 Properties of Liquids Chemistry LibreTexts What Would Be The Boiling Point Of Pure Water the boiling point of a liquid varies according to the applied pressure; if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere. for instance, at a standard atmospheric pressure, pure water has a fixed boiling point. The boiling point of water. What Would Be The Boiling Point Of Pure Water.
From www.dreamstime.com
Boiling Point of Water in Different Altitude Meter Levels Outline What Would Be The Boiling Point Of Pure Water The boiling point of water is 99.97 °c, or 211.95 °f , under standard pressure at sea level. the boiling point of a liquid varies according to the applied pressure; Usually, you'll find that these values are. The standard boiling point of water is 99.61 °c at 1 bar of pressure. for instance, at a standard atmospheric pressure,. What Would Be The Boiling Point Of Pure Water.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation What Would Be The Boiling Point Of Pure Water However, if impurities such as sodium chloride or the common. The boiling point of water is 99.97 °c, or 211.95 °f , under standard pressure at sea level. what’s the boiling point of water? the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. Usually, you'll find. What Would Be The Boiling Point Of Pure Water.
From pakmcqs.com
Boiling point of pure water is __________? PakMcqs What Would Be The Boiling Point Of Pure Water the boiling point is the temperature at which a liquid substance changes to its gaseous phase. what’s the boiling point of water? However, if impurities such as sodium chloride or the common. the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. Usually, you'll find that. What Would Be The Boiling Point Of Pure Water.
From www.tes.com
Pure and Impure GCSE AQA Teaching Resources What Would Be The Boiling Point Of Pure Water The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. The normal boiling point is the temperature at which the vapour pressure is equal. the boiling point is the temperature at which a liquid substance changes to its gaseous phase. Usually, you'll find that these. What Would Be The Boiling Point Of Pure Water.
From manuallistintermits.z19.web.core.windows.net
How To Read Phase Diagrams Chemistry What Would Be The Boiling Point Of Pure Water the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere. The boiling point of water is 99.97 °c, or 211.95 °f. What Would Be The Boiling Point Of Pure Water.
From 88guru.com
Melting Point of Ice and Boiling Point of Water 88Guru What Would Be The Boiling Point Of Pure Water the boiling point is the temperature at which a liquid substance changes to its gaseous phase. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere. what’s the boiling point of water? for instance, at a standard atmospheric pressure, pure. What Would Be The Boiling Point Of Pure Water.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Would Be The Boiling Point Of Pure Water for instance, at a standard atmospheric pressure, pure water has a fixed boiling point. the boiling point is the temperature at which a liquid substance changes to its gaseous phase. The boiling point of water is 99.97 °c, or 211.95 °f , under standard pressure at sea level. The normal boiling point is the temperature at which the. What Would Be The Boiling Point Of Pure Water.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Would Be The Boiling Point Of Pure Water what’s the boiling point of water? The standard boiling point of water is 99.61 °c at 1 bar of pressure. The normal boiling point is the temperature at which the vapour pressure is equal. for instance, at a standard atmospheric pressure, pure water has a fixed boiling point. The standard boiling point, as defined by the iupac in. What Would Be The Boiling Point Of Pure Water.
From schematicfritolay40sdrtk.z13.web.core.windows.net
T V Diagram Of Water What Would Be The Boiling Point Of Pure Water However, if impurities such as sodium chloride or the common. the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The normal boiling point is the temperature at which the vapour pressure is equal. the boiling point of a liquid varies according to the applied pressure; . What Would Be The Boiling Point Of Pure Water.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock What Would Be The Boiling Point Of Pure Water for instance, at a standard atmospheric pressure, pure water has a fixed boiling point. the boiling point of a liquid varies according to the applied pressure; The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. The boiling point of water is 99.97 °c,. What Would Be The Boiling Point Of Pure Water.
From www.chegg.com
Solved suppose the boiling point of pure water at h h What Would Be The Boiling Point Of Pure Water for instance, at a standard atmospheric pressure, pure water has a fixed boiling point. the boiling point of a liquid varies according to the applied pressure; if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere. The standard boiling point of. What Would Be The Boiling Point Of Pure Water.
From www.youtube.com
CHEMISTRY 201 Calculating Boiling Point Using Clausius Clapeyron What Would Be The Boiling Point Of Pure Water However, if impurities such as sodium chloride or the common. The boiling point of water is 99.97 °c, or 211.95 °f , under standard pressure at sea level. The normal boiling point is the temperature at which the vapour pressure is equal. the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of. What Would Be The Boiling Point Of Pure Water.
From www.researchgate.net
Extraction at boiling point of solvents Download Scientific Diagram What Would Be The Boiling Point Of Pure Water The normal boiling point is the temperature at which the vapour pressure is equal. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere. for instance, at a standard atmospheric pressure, pure water has a fixed boiling point. The standard boiling point. What Would Be The Boiling Point Of Pure Water.
From askfilo.com
The normal boiling point of pure water is 373.15 K. Calculate the boiling.. What Would Be The Boiling Point Of Pure Water However, if impurities such as sodium chloride or the common. The normal boiling point is the temperature at which the vapour pressure is equal. the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. what’s the boiling point of water? the boiling point is the temperature. What Would Be The Boiling Point Of Pure Water.
From www.animalia-life.club
Boiling Point Of Water For Kids What Would Be The Boiling Point Of Pure Water if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere. the boiling point is the temperature at which a liquid substance changes to its gaseous phase. The normal boiling point is the temperature at which the vapour pressure is equal. The standard. What Would Be The Boiling Point Of Pure Water.
From www.chegg.com
Solved Consider the following aqueous solutions.\\nSolution What Would Be The Boiling Point Of Pure Water the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere. the boiling point is the temperature at which a liquid. What Would Be The Boiling Point Of Pure Water.
From www.chegg.com
Solved Arrange the boiling points of the aqueous solutions, What Would Be The Boiling Point Of Pure Water what’s the boiling point of water? The normal boiling point is the temperature at which the vapour pressure is equal. the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The standard boiling point of water is 99.61 °c at 1 bar of pressure. The standard boiling. What Would Be The Boiling Point Of Pure Water.
From pressbooks.online.ucf.edu
12.5 Colligative Properties Chemistry Fundamentals What Would Be The Boiling Point Of Pure Water However, if impurities such as sodium chloride or the common. the boiling point is the temperature at which a liquid substance changes to its gaseous phase. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. the normal boiling point or the atmospheric boiling. What Would Be The Boiling Point Of Pure Water.
From water-purifiers.com
How Pure Is Your Boiling Water What Would Be The Boiling Point Of Pure Water the boiling point is the temperature at which a liquid substance changes to its gaseous phase. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere. Usually, you'll find that these values are. The boiling point of water is 99.97 °c, or. What Would Be The Boiling Point Of Pure Water.
From snipe.fm
😍 How does salt affect boiling point. Why Does Adding Salt Increase the What Would Be The Boiling Point Of Pure Water The boiling point of water is 99.97 °c, or 211.95 °f , under standard pressure at sea level. The standard boiling point of water is 99.61 °c at 1 bar of pressure. the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. However, if impurities such as sodium. What Would Be The Boiling Point Of Pure Water.
From www.tes.com
Mixtures and Purity GCSE Lesson (SC2a CC2a) Pure or Impure? Teaching What Would Be The Boiling Point Of Pure Water the boiling point is the temperature at which a liquid substance changes to its gaseous phase. what’s the boiling point of water? The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. the boiling point of a liquid varies according to the applied. What Would Be The Boiling Point Of Pure Water.
From ar.inspiredpencil.com
Boiling Point Of Water Examples What Would Be The Boiling Point Of Pure Water The boiling point of water is 99.97 °c, or 211.95 °f , under standard pressure at sea level. the boiling point of a liquid varies according to the applied pressure; if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere. However, if. What Would Be The Boiling Point Of Pure Water.
From ar.inspiredpencil.com
Boiling Point Of Water Examples What Would Be The Boiling Point Of Pure Water the boiling point is the temperature at which a liquid substance changes to its gaseous phase. The boiling point of water is 99.97 °c, or 211.95 °f , under standard pressure at sea level. for instance, at a standard atmospheric pressure, pure water has a fixed boiling point. Usually, you'll find that these values are. if you. What Would Be The Boiling Point Of Pure Water.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 What Would Be The Boiling Point Of Pure Water However, if impurities such as sodium chloride or the common. what’s the boiling point of water? Usually, you'll find that these values are. the normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The boiling point of water is 99.97 °c, or 211.95 °f , under standard. What Would Be The Boiling Point Of Pure Water.
From www.youtube.com
Method To Determine Boiling Point Of A Liquid Basic Principles and What Would Be The Boiling Point Of Pure Water for instance, at a standard atmospheric pressure, pure water has a fixed boiling point. Usually, you'll find that these values are. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. The boiling point of water is 99.97 °c, or 211.95 °f , under standard. What Would Be The Boiling Point Of Pure Water.