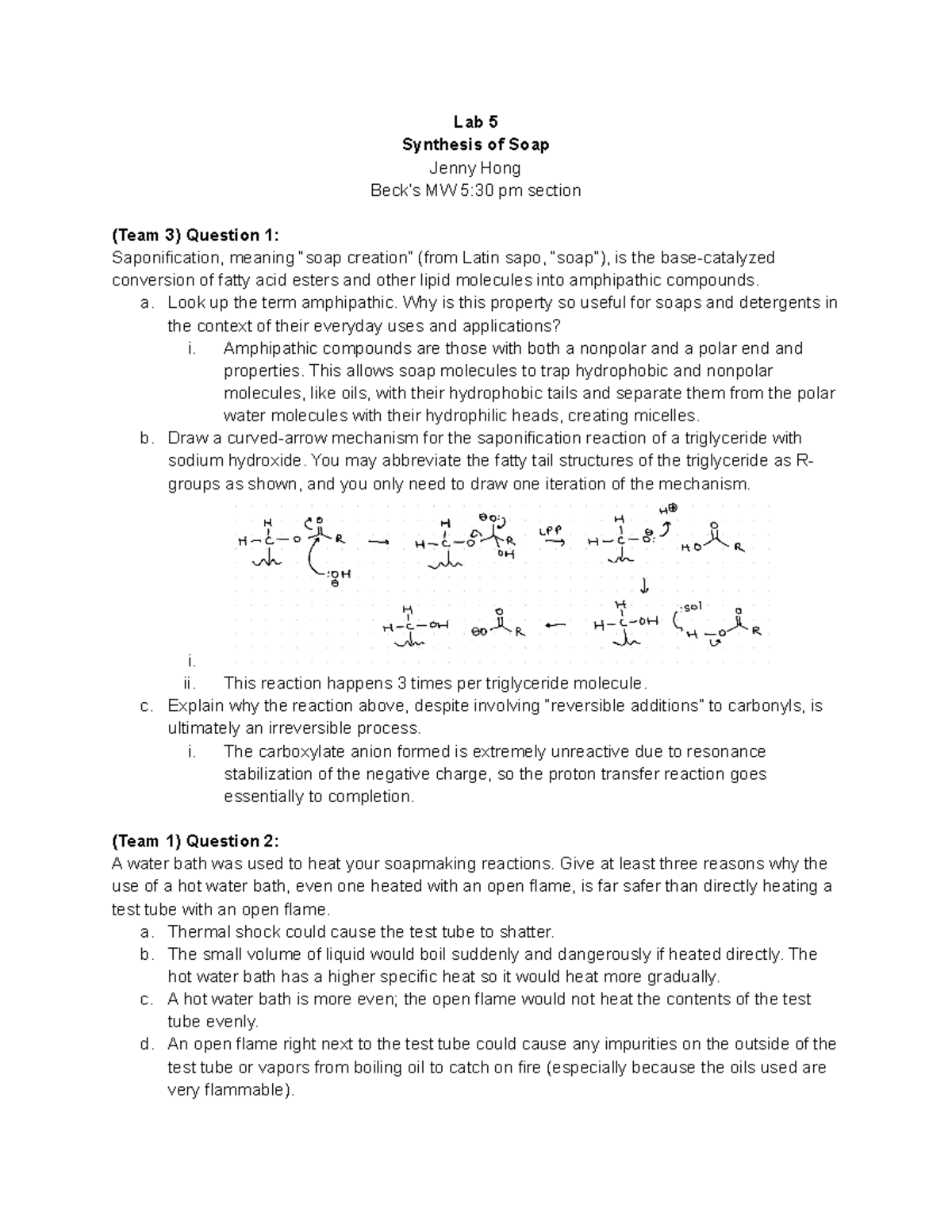
Soap Lab Answers . soaps are carboxylate salts with very long hydrocarbon chains. the objective of this laboratory is to make lye soap via the saponification reaction. Draw an emulsified oil in a micelle. stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as soaps. to make soap, we will perform a hydrolysis reaction on olive oil to separate the glycerol from the fatty acids (figure \(\pageindex{3}\)). 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap. Explain how soaps emulsify oils from your skin. To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of. soap lab flashcards | quizlet. The ancient roman tradition called for mixing rain. Soap can be made from the base hydrolysis of a fat or an oil.
from www.studocu.com
soaps are carboxylate salts with very long hydrocarbon chains. 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap. Draw an emulsified oil in a micelle. To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of. soap lab flashcards | quizlet. The ancient roman tradition called for mixing rain. Soap can be made from the base hydrolysis of a fat or an oil. Explain how soaps emulsify oils from your skin. stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as soaps. to make soap, we will perform a hydrolysis reaction on olive oil to separate the glycerol from the fatty acids (figure \(\pageindex{3}\)).
Lab 5 Synthesis of soap lab assignment 5 Lab 5 Synthesis of Soap
Soap Lab Answers to make soap, we will perform a hydrolysis reaction on olive oil to separate the glycerol from the fatty acids (figure \(\pageindex{3}\)). Draw an emulsified oil in a micelle. Explain how soaps emulsify oils from your skin. To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of. The ancient roman tradition called for mixing rain. Soap can be made from the base hydrolysis of a fat or an oil. soap lab flashcards | quizlet. soaps are carboxylate salts with very long hydrocarbon chains. 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap. stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as soaps. the objective of this laboratory is to make lye soap via the saponification reaction. to make soap, we will perform a hydrolysis reaction on olive oil to separate the glycerol from the fatty acids (figure \(\pageindex{3}\)).
From www.thinkswap.com
How to Make an Effective Soap Chemistry Year 11 SACE Thinkswap Soap Lab Answers stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as soaps. Draw an emulsified oil in a micelle. soap lab flashcards | quizlet. 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap. the objective of this laboratory is to make lye. Soap Lab Answers.
From studylib.net
Making and Testing Soap Lab Soap Lab Answers 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap. soaps are carboxylate salts with very long hydrocarbon chains. soap lab flashcards | quizlet. To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of. Explain how soaps emulsify oils from your skin.. Soap Lab Answers.
From www.docsity.com
Soap Lab Study notes Chemical Experimentation Docsity Soap Lab Answers soap lab flashcards | quizlet. The ancient roman tradition called for mixing rain. stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as soaps. the objective of this laboratory is to make lye soap via the saponification reaction. 5.0 (2 reviews) write the chemical equation for the. Soap Lab Answers.
From www.studocu.com
Soap prelab The goals of this lab are to make soap, test the Soap Lab Answers Explain how soaps emulsify oils from your skin. Soap can be made from the base hydrolysis of a fat or an oil. the objective of this laboratory is to make lye soap via the saponification reaction. Draw an emulsified oil in a micelle. soap lab flashcards | quizlet. soaps are carboxylate salts with very long hydrocarbon chains.. Soap Lab Answers.
From www.chegg.com
Solved REPORT SHEET Saponification and Soaps A. Soap Lab Answers stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as soaps. soaps are carboxylate salts with very long hydrocarbon chains. the objective of this laboratory is to make lye soap via the saponification reaction. soap lab flashcards | quizlet. Soap can be made from the base. Soap Lab Answers.
From www.chegg.com
Solved Assignment for Lab 6 Synthesis of Soap What Soap Lab Answers 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap. To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of. soap lab flashcards | quizlet. Soap can be made from the base hydrolysis of a fat or an oil. Draw an emulsified oil. Soap Lab Answers.
From www.flinnsci.ca
Making Soap The Oldest Organic Reaction—ChemTopic™ Lab Activity Soap Lab Answers the objective of this laboratory is to make lye soap via the saponification reaction. stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as soaps. The ancient roman tradition called for mixing rain. Soap can be made from the base hydrolysis of a fat or an oil. 5.0. Soap Lab Answers.
From studylib.net
Soap Lab Soap Lab Answers Explain how soaps emulsify oils from your skin. Draw an emulsified oil in a micelle. to make soap, we will perform a hydrolysis reaction on olive oil to separate the glycerol from the fatty acids (figure \(\pageindex{3}\)). soap lab flashcards | quizlet. the objective of this laboratory is to make lye soap via the saponification reaction. . Soap Lab Answers.
From www.chegg.com
Solved B. Properties of Soaps and Detergents Tests Lab Soap Soap Lab Answers stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as soaps. soaps are carboxylate salts with very long hydrocarbon chains. Draw an emulsified oil in a micelle. To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of.. Soap Lab Answers.
From www.chegg.com
Solved Soap Lab Assign... out References Mailings Review Soap Lab Answers Soap can be made from the base hydrolysis of a fat or an oil. 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap. soaps are carboxylate salts with very long hydrocarbon chains. stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as. Soap Lab Answers.
From www.chegg.com
B. Properties of Soaps and Detergents Tests Lab Soap Soap Lab Answers Explain how soaps emulsify oils from your skin. The ancient roman tradition called for mixing rain. stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as soaps. Soap can be made from the base hydrolysis of a fat or an oil. To prepare soap by alkaline hydrolysis (saponification) of. Soap Lab Answers.
From www.chegg.com
Lab 8 Lab 8 Preparation and Properties of Soap Team Soap Lab Answers To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of. 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap. soaps are carboxylate salts with very long hydrocarbon chains. soap lab flashcards | quizlet. the objective of this laboratory is to. Soap Lab Answers.
From www.coursehero.com
Can you answer to this soap making lab? Questions Type written Soap Lab Answers the objective of this laboratory is to make lye soap via the saponification reaction. Explain how soaps emulsify oils from your skin. soaps are carboxylate salts with very long hydrocarbon chains. To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of. to make soap, we will. Soap Lab Answers.
From www.studocu.com
13Saponification lab report Experiment 13 Preparation of Soap Soap Lab Answers to make soap, we will perform a hydrolysis reaction on olive oil to separate the glycerol from the fatty acids (figure \(\pageindex{3}\)). 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap. Draw an emulsified oil in a micelle. To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the. Soap Lab Answers.
From www.chegg.com
Solved Experiment 7 Lab Report MAKING SOAP THE Soap Lab Answers Draw an emulsified oil in a micelle. the objective of this laboratory is to make lye soap via the saponification reaction. soap lab flashcards | quizlet. Soap can be made from the base hydrolysis of a fat or an oil. to make soap, we will perform a hydrolysis reaction on olive oil to separate the glycerol from. Soap Lab Answers.
From www.numerade.com
SOLVED Prelab Assignment for Lab Synthesis of Soap What information Soap Lab Answers The ancient roman tradition called for mixing rain. Explain how soaps emulsify oils from your skin. To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of. the objective of this laboratory is to make lye soap via the saponification reaction. to make soap, we will perform a. Soap Lab Answers.
From www.studocu.com
Soap Lab Report BSEE 14 Chemistry for Engineers Experiment 3 Making Soap Lab Answers Draw an emulsified oil in a micelle. to make soap, we will perform a hydrolysis reaction on olive oil to separate the glycerol from the fatty acids (figure \(\pageindex{3}\)). the objective of this laboratory is to make lye soap via the saponification reaction. 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap.. Soap Lab Answers.
From www.numerade.com
SOLVED Esterification and Making Soap Post Lab Questions (6 points Soap Lab Answers stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as soaps. Soap can be made from the base hydrolysis of a fat or an oil. Explain how soaps emulsify oils from your skin. soaps are carboxylate salts with very long hydrocarbon chains. To prepare soap by alkaline hydrolysis. Soap Lab Answers.
From www.chegg.com
Lab 8 Preparation and Properties of Soap Team Soap Lab Answers The ancient roman tradition called for mixing rain. the objective of this laboratory is to make lye soap via the saponification reaction. soaps are carboxylate salts with very long hydrocarbon chains. Draw an emulsified oil in a micelle. soap lab flashcards | quizlet. Explain how soaps emulsify oils from your skin. stearic (animal fat), lauric (vegetable. Soap Lab Answers.
From www.youtube.com
Chemistry of Soap Lab demo YouTube Soap Lab Answers soap lab flashcards | quizlet. Draw an emulsified oil in a micelle. soaps are carboxylate salts with very long hydrocarbon chains. to make soap, we will perform a hydrolysis reaction on olive oil to separate the glycerol from the fatty acids (figure \(\pageindex{3}\)). Soap can be made from the base hydrolysis of a fat or an oil.. Soap Lab Answers.
From www.chegg.com
Preparation for Soap Lab A. Emulsifying Properties Soap Lab Answers stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as soaps. Draw an emulsified oil in a micelle. The ancient roman tradition called for mixing rain. soaps are carboxylate salts with very long hydrocarbon chains. 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and. Soap Lab Answers.
From www.chegg.com
Solved Name Section Preparation and Properties of Soap Soap Lab Answers 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap. To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of. to make soap, we will perform a hydrolysis reaction on olive oil to separate the glycerol from the fatty acids (figure \(\pageindex{3}\)). . Soap Lab Answers.
From www.studocu.com
Soap Lab V3 Chemistry The Soapmaking Factory Lab Overview In this Soap Lab Answers soap lab flashcards | quizlet. stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as soaps. 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap. to make soap, we will perform a hydrolysis reaction on olive oil to separate the glycerol. Soap Lab Answers.
From www.chegg.com
Lab 8 Preparation and Properties of Soap Team Soap Lab Answers the objective of this laboratory is to make lye soap via the saponification reaction. 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap. soaps are carboxylate salts with very long hydrocarbon chains. to make soap, we will perform a hydrolysis reaction on olive oil to separate the glycerol from the fatty. Soap Lab Answers.
From www.chegg.com
Solved Seiko Team LAB REPORT SHEET Saponification and Soaps Soap Lab Answers to make soap, we will perform a hydrolysis reaction on olive oil to separate the glycerol from the fatty acids (figure \(\pageindex{3}\)). the objective of this laboratory is to make lye soap via the saponification reaction. Explain how soaps emulsify oils from your skin. The ancient roman tradition called for mixing rain. 5.0 (2 reviews) write the chemical. Soap Lab Answers.
From www.chegg.com
Solved STOICHIOMETRY SYNTHESIS OF SOAP AND ITS PROPERTIES Soap Lab Answers stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as soaps. soaps are carboxylate salts with very long hydrocarbon chains. Explain how soaps emulsify oils from your skin. the objective of this laboratory is to make lye soap via the saponification reaction. To prepare soap by alkaline. Soap Lab Answers.
From www.chegg.com
Solved Post Lab Questions 1. If the soap solution had a Soap Lab Answers soap lab flashcards | quizlet. The ancient roman tradition called for mixing rain. Explain how soaps emulsify oils from your skin. the objective of this laboratory is to make lye soap via the saponification reaction. Soap can be made from the base hydrolysis of a fat or an oil. Draw an emulsified oil in a micelle. stearic. Soap Lab Answers.
From www.chegg.com
Solved Commercial Detergent Test Labprepared Soap Soap Lab Answers to make soap, we will perform a hydrolysis reaction on olive oil to separate the glycerol from the fatty acids (figure \(\pageindex{3}\)). To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of. Soap can be made from the base hydrolysis of a fat or an oil. stearic. Soap Lab Answers.
From www.studocu.com
Lab 5 Synthesis of soap lab assignment 5 Lab 5 Synthesis of Soap Soap Lab Answers the objective of this laboratory is to make lye soap via the saponification reaction. soap lab flashcards | quizlet. stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as soaps. Draw an emulsified oil in a micelle. To prepare soap by alkaline hydrolysis (saponification) of natural fats. Soap Lab Answers.
From www.chegg.com
Solved write a formal lab soap report. please don't use the Soap Lab Answers Draw an emulsified oil in a micelle. the objective of this laboratory is to make lye soap via the saponification reaction. to make soap, we will perform a hydrolysis reaction on olive oil to separate the glycerol from the fatty acids (figure \(\pageindex{3}\)). 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap.. Soap Lab Answers.
From www.studocu.com
Orgo Soap Lab Key Chem 546 Lab 10 Preparation of Soap from Soap Lab Answers 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap. the objective of this laboratory is to make lye soap via the saponification reaction. Soap can be made from the base hydrolysis of a fat or an oil. soaps are carboxylate salts with very long hydrocarbon chains. To prepare soap by alkaline hydrolysis. Soap Lab Answers.
From classful.com
Making Soap Lab Demonstration Guide Exploring Saponification Classful Soap Lab Answers Soap can be made from the base hydrolysis of a fat or an oil. The ancient roman tradition called for mixing rain. soaps are carboxylate salts with very long hydrocarbon chains. the objective of this laboratory is to make lye soap via the saponification reaction. soap lab flashcards | quizlet. Explain how soaps emulsify oils from your. Soap Lab Answers.
From www.scribd.com
Soap Lab Report by AD Organic Chemistry Chemistry Soap Lab Answers Soap can be made from the base hydrolysis of a fat or an oil. stearic (animal fat), lauric (vegetable oil) and palmitic (palm oil) are a few sodium fatty acid that are known as soaps. To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of. to make. Soap Lab Answers.
From www.chegg.com
Solved REPORT SHEET LAB Saponification And Soaps 32 A. Sa... Soap Lab Answers Soap can be made from the base hydrolysis of a fat or an oil. To prepare soap by alkaline hydrolysis (saponification) of natural fats and test some of the chemical properties and cleansing power of. soaps are carboxylate salts with very long hydrocarbon chains. 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap.. Soap Lab Answers.
From dxoxvgvjf.blob.core.windows.net
Liquid Soap Lab Report at Abigail Newkirk blog Soap Lab Answers Soap can be made from the base hydrolysis of a fat or an oil. soaps are carboxylate salts with very long hydrocarbon chains. Explain how soaps emulsify oils from your skin. 5.0 (2 reviews) write the chemical equation for the reaction between mgcl2 and the soap. to make soap, we will perform a hydrolysis reaction on olive oil. Soap Lab Answers.