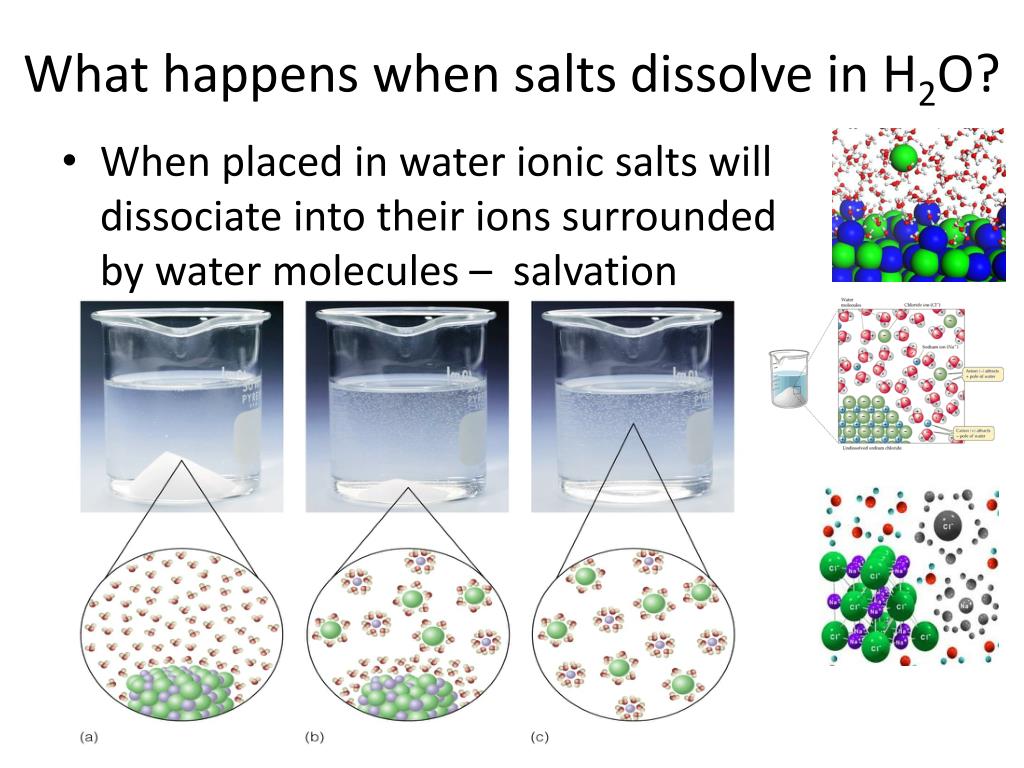
Does Salt Dissolve In Acetone . 83 g of licl dissolve in 100 ml of water at 20°c, but only about 4.1 g of licl dissolve in 100 ml of acetone. For a solution, this point is called the saturation point and the solution itself is called a saturated solution. For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility in water. In fact, the opposite is true: Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. A salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. As you would almost certainly predict, especially if you’ve ever inadvertently taken a. A salt is insoluble if the concentration of an. At the point of saturation, no more. The first substance is table salt, or sodium chloride. Sodium chloride and sodium bromide being less soluble in acetone get precipitated from the solution and can be. When ionic compounds dissolve in water, the individual ions. Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently polar to be completely miscible with water yet sufficiently nonpolar to be completely miscible with all.
from www.slideserve.com
When ionic compounds dissolve in water, the individual ions. As you would almost certainly predict, especially if you’ve ever inadvertently taken a. At the point of saturation, no more. For a solution, this point is called the saturation point and the solution itself is called a saturated solution. 83 g of licl dissolve in 100 ml of water at 20°c, but only about 4.1 g of licl dissolve in 100 ml of acetone. Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently polar to be completely miscible with water yet sufficiently nonpolar to be completely miscible with all. For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility in water. A salt is insoluble if the concentration of an. In fact, the opposite is true: Sodium chloride and sodium bromide being less soluble in acetone get precipitated from the solution and can be.
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free
Does Salt Dissolve In Acetone For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility in water. As you would almost certainly predict, especially if you’ve ever inadvertently taken a. The first substance is table salt, or sodium chloride. Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility in water. A salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. At the point of saturation, no more. A salt is insoluble if the concentration of an. Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently polar to be completely miscible with water yet sufficiently nonpolar to be completely miscible with all. For a solution, this point is called the saturation point and the solution itself is called a saturated solution. 83 g of licl dissolve in 100 ml of water at 20°c, but only about 4.1 g of licl dissolve in 100 ml of acetone. When ionic compounds dissolve in water, the individual ions. Sodium chloride and sodium bromide being less soluble in acetone get precipitated from the solution and can be. In fact, the opposite is true:
From www.paintspraypro.com
Mineral Spirits Vs Acetone Pick the Right One for Your DIY Does Salt Dissolve In Acetone When ionic compounds dissolve in water, the individual ions. In fact, the opposite is true: A salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. 83 g of licl dissolve in 100 ml of water at 20°c, but only about 4.1 g of licl. Does Salt Dissolve In Acetone.
From www.youtube.com
Why does acetone dissolve both polar and nonpolar? YouTube Does Salt Dissolve In Acetone The first substance is table salt, or sodium chloride. A salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. When ionic compounds dissolve in water, the individual ions. Sodium chloride and sodium bromide being less soluble in acetone get precipitated from the solution and. Does Salt Dissolve In Acetone.
From www.youtube.com
How Salt Dissolves in Water? YouTube Does Salt Dissolve In Acetone Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. At the point of saturation, no more. For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility in water. A salt is insoluble if the concentration of an. When ionic compounds dissolve in water, the. Does Salt Dissolve In Acetone.
From proper-cooking.info
Acetone Lewis Structure With Polarity Does Salt Dissolve In Acetone As you would almost certainly predict, especially if you’ve ever inadvertently taken a. For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility in water. When ionic compounds dissolve in water, the individual ions. Sodium chloride and sodium bromide being less soluble in acetone get precipitated from the solution and can be. For. Does Salt Dissolve In Acetone.
From www.youtube.com
Dissolving Styrofoam With Acetone Experiment YouTube Does Salt Dissolve In Acetone 83 g of licl dissolve in 100 ml of water at 20°c, but only about 4.1 g of licl dissolve in 100 ml of acetone. In fact, the opposite is true: Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently polar to be completely miscible with water yet sufficiently nonpolar to be completely miscible with all. For example, acetone. Does Salt Dissolve In Acetone.
From www.youtube.com
Dissolving Polystyrene In Acetone YouTube Does Salt Dissolve In Acetone The first substance is table salt, or sodium chloride. For a solution, this point is called the saturation point and the solution itself is called a saturated solution. Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. For example, acetone is a highly polar organic molecule, so it's not surprising it. Does Salt Dissolve In Acetone.
From www.labkafe.com
Solubility of Salts ‒ Why Common Salts are So Soluble in Water Labkafe Does Salt Dissolve In Acetone Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. A salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. Sodium chloride and sodium bromide being less soluble in acetone get precipitated from the solution and. Does Salt Dissolve In Acetone.
From study.com
Reactions of Acetone with Water, Alcohol & Iodine Lesson Does Salt Dissolve In Acetone When ionic compounds dissolve in water, the individual ions. Sodium chloride and sodium bromide being less soluble in acetone get precipitated from the solution and can be. As you would almost certainly predict, especially if you’ve ever inadvertently taken a. For a solution, this point is called the saturation point and the solution itself is called a saturated solution. Salt. Does Salt Dissolve In Acetone.
From www.vectorstock.com
How does sodium chloride nacl dissolve in water Vector Image Does Salt Dissolve In Acetone When ionic compounds dissolve in water, the individual ions. For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility in water. At the point of saturation, no more. Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. The first substance is table salt, or. Does Salt Dissolve In Acetone.
From www.slideserve.com
PPT BASIC’S OF SOLUTION CHEMISTRY PowerPoint Presentation, free Does Salt Dissolve In Acetone For a solution, this point is called the saturation point and the solution itself is called a saturated solution. At the point of saturation, no more. Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. A salt is insoluble if the concentration of an. 83 g of licl dissolve in 100. Does Salt Dissolve In Acetone.
From www.pinterest.ca
This salt fractionation using acetone (dyed blue) and the higher Does Salt Dissolve In Acetone 83 g of licl dissolve in 100 ml of water at 20°c, but only about 4.1 g of licl dissolve in 100 ml of acetone. A salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. As you would almost certainly predict, especially if you’ve. Does Salt Dissolve In Acetone.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID762021 Does Salt Dissolve In Acetone Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently polar to be completely miscible with water yet sufficiently nonpolar to be completely miscible with all. When ionic compounds dissolve in water, the individual ions. At the point of saturation, no more. A salt is insoluble if the concentration of an. Sodium chloride and sodium bromide being less soluble in. Does Salt Dissolve In Acetone.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik Does Salt Dissolve In Acetone Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently polar to be completely miscible with water yet sufficiently nonpolar to be completely miscible with all. When ionic compounds dissolve in water, the individual ions. For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility in water. A salt is insoluble if the. Does Salt Dissolve In Acetone.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Does Salt Dissolve In Acetone As you would almost certainly predict, especially if you’ve ever inadvertently taken a. The first substance is table salt, or sodium chloride. In fact, the opposite is true: For a solution, this point is called the saturation point and the solution itself is called a saturated solution. A salt is insoluble if the concentration of an. Organic liquids such as. Does Salt Dissolve In Acetone.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Does Salt Dissolve In Acetone For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility in water. Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. 83 g of licl dissolve in 100 ml of water at 20°c, but only about 4.1 g of licl dissolve in 100 ml. Does Salt Dissolve In Acetone.
From www.slideserve.com
PPT Introductory Chemistry , 3 rd Edition Nivaldo Tro PowerPoint Does Salt Dissolve In Acetone The first substance is table salt, or sodium chloride. For a solution, this point is called the saturation point and the solution itself is called a saturated solution. At the point of saturation, no more. When ionic compounds dissolve in water, the individual ions. In fact, the opposite is true: Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently. Does Salt Dissolve In Acetone.
From www.examanalysis.in
Acids, Bases and Salts Does Salt Dissolve In Acetone The first substance is table salt, or sodium chloride. Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently polar to be completely miscible with water yet sufficiently nonpolar to be completely miscible with all. A salt is insoluble if the concentration of an. At the point of saturation, no more. For example, acetone is a highly polar organic molecule,. Does Salt Dissolve In Acetone.
From www.youtube.com
a mixture of ethanol and acetone shows positive deviation YouTube Does Salt Dissolve In Acetone As you would almost certainly predict, especially if you’ve ever inadvertently taken a. For a solution, this point is called the saturation point and the solution itself is called a saturated solution. When ionic compounds dissolve in water, the individual ions. The first substance is table salt, or sodium chloride. Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently. Does Salt Dissolve In Acetone.
From www.youtube.com
Acetone Dissolving Styrofoam Polystyrene YouTube Does Salt Dissolve In Acetone 83 g of licl dissolve in 100 ml of water at 20°c, but only about 4.1 g of licl dissolve in 100 ml of acetone. A salt is insoluble if the concentration of an. Sodium chloride and sodium bromide being less soluble in acetone get precipitated from the solution and can be. For example, acetone is a highly polar organic. Does Salt Dissolve In Acetone.
From ar.inspiredpencil.com
Styrofoam And Acetone Chemical Reaction Does Salt Dissolve In Acetone Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently polar to be completely miscible with water yet sufficiently nonpolar to be completely miscible with all. At the point of saturation, no more. For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility in water. When ionic compounds dissolve in water, the individual. Does Salt Dissolve In Acetone.
From www.youtube.com
Is acetone polar or nonpolar? Will NaCl dissolve in it? YouTube Does Salt Dissolve In Acetone For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility in water. Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently polar to be completely miscible with water yet sufficiently nonpolar to be completely miscible with all. 83 g of licl dissolve in 100 ml of water at 20°c, but only about. Does Salt Dissolve In Acetone.
From www.chegg.com
Solved You notice that NaCl salt easily solubles in water, Does Salt Dissolve In Acetone The first substance is table salt, or sodium chloride. When ionic compounds dissolve in water, the individual ions. Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently polar to be completely miscible with water yet sufficiently nonpolar to be completely miscible with all. For example, acetone is a highly polar organic molecule, so it's not surprising it has high. Does Salt Dissolve In Acetone.
From slideplayer.com
DO NOW Turn in Solubility Curves and Solutions handout ppt download Does Salt Dissolve In Acetone Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently polar to be completely miscible with water yet sufficiently nonpolar to be completely miscible with all. For a solution, this point is called the saturation point and the solution itself is called a saturated solution. For example, acetone is a highly polar organic molecule, so it's not surprising it has. Does Salt Dissolve In Acetone.
From www.youtube.com
Salt dissolves in water Is this a chemical or physical change? YouTube Does Salt Dissolve In Acetone 83 g of licl dissolve in 100 ml of water at 20°c, but only about 4.1 g of licl dissolve in 100 ml of acetone. Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently polar to be completely miscible with water yet sufficiently nonpolar to be completely miscible with all. For a solution, this point is called the saturation. Does Salt Dissolve In Acetone.
From www.youtube.com
GCSE CHEMISTRY ACIDS AND BASES LESSON 19 salts solubility YouTube Does Salt Dissolve In Acetone A salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. A salt is insoluble if the concentration of an. 83 g of licl dissolve in 100 ml of water at 20°c, but only about 4.1 g of licl dissolve in 100 ml of acetone.. Does Salt Dissolve In Acetone.
From www.researchgate.net
stirrer extraction Soxhlet Extraction Acetone Acetone Does Salt Dissolve In Acetone A salt is insoluble if the concentration of an. For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility in water. Sodium chloride and sodium bromide being less soluble in acetone get precipitated from the solution and can be. Salt is an ionic compound, meaning it is a substance made up of electrically. Does Salt Dissolve In Acetone.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt Does Salt Dissolve In Acetone For a solution, this point is called the saturation point and the solution itself is called a saturated solution. In fact, the opposite is true: Sodium chloride and sodium bromide being less soluble in acetone get precipitated from the solution and can be. For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility. Does Salt Dissolve In Acetone.
From ecurrencythailand.com
Which Liquid Dissolves Aspirin The Fastest? 10 Most Correct Answers Does Salt Dissolve In Acetone As you would almost certainly predict, especially if you’ve ever inadvertently taken a. At the point of saturation, no more. For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility in water. In fact, the opposite is true: 83 g of licl dissolve in 100 ml of water at 20°c, but only about. Does Salt Dissolve In Acetone.
From www.chemistrysteps.com
Solubility of Organic Compounds Chemistry Steps Does Salt Dissolve In Acetone The first substance is table salt, or sodium chloride. As you would almost certainly predict, especially if you’ve ever inadvertently taken a. At the point of saturation, no more. Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently polar to be completely miscible with water yet sufficiently nonpolar to be completely miscible with all. For a solution, this point. Does Salt Dissolve In Acetone.
From slideplayer.com
Physical and Chemical Changes ppt download Does Salt Dissolve In Acetone At the point of saturation, no more. For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility in water. A salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. Sodium chloride and sodium bromide being less soluble. Does Salt Dissolve In Acetone.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Does Salt Dissolve In Acetone For example, acetone is a highly polar organic molecule, so it's not surprising it has high solubility/mixibility in water. For a solution, this point is called the saturation point and the solution itself is called a saturated solution. When ionic compounds dissolve in water, the individual ions. Sodium chloride and sodium bromide being less soluble in acetone get precipitated from. Does Salt Dissolve In Acetone.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Does Salt Dissolve In Acetone For a solution, this point is called the saturation point and the solution itself is called a saturated solution. The first substance is table salt, or sodium chloride. Sodium chloride and sodium bromide being less soluble in acetone get precipitated from the solution and can be. 83 g of licl dissolve in 100 ml of water at 20°c, but only. Does Salt Dissolve In Acetone.
From www.shalom-education.com
Ionic Compounds Edexcel GCSE Chemistry Revision Does Salt Dissolve In Acetone For a solution, this point is called the saturation point and the solution itself is called a saturated solution. A salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. In fact, the opposite is true: 83 g of licl dissolve in 100 ml of. Does Salt Dissolve In Acetone.
From www.transtutors.com
(Get Answer) 1. SODIUM IODIDE IN ACETONE Tone, With A Dielectric Does Salt Dissolve In Acetone 83 g of licl dissolve in 100 ml of water at 20°c, but only about 4.1 g of licl dissolve in 100 ml of acetone. In fact, the opposite is true: A salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. For a solution,. Does Salt Dissolve In Acetone.
From www.slideserve.com
PPT Chapter 7 Chemical Reactions PowerPoint Presentation, free Does Salt Dissolve In Acetone Organic liquids such as acetone, ethanol, and tetrahydrofuran are sufficiently polar to be completely miscible with water yet sufficiently nonpolar to be completely miscible with all. The first substance is table salt, or sodium chloride. At the point of saturation, no more. In fact, the opposite is true: Sodium chloride and sodium bromide being less soluble in acetone get precipitated. Does Salt Dissolve In Acetone.