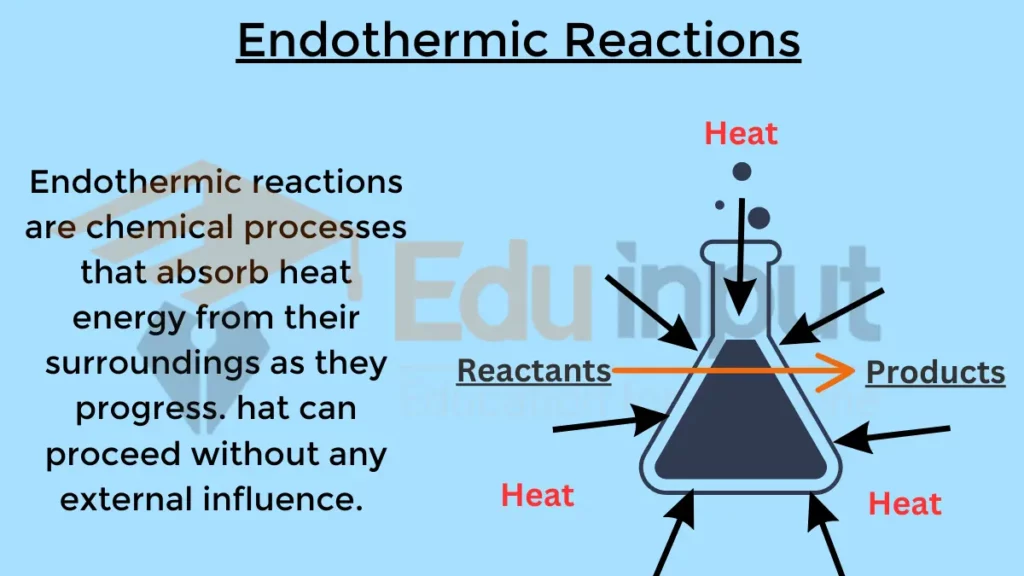
Endothermic Reaction General Formula . an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total internal energy level or enthalpy. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. use bond dissociation energies to calculate enthalpy change or heat of reaction. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. Determine if a chemical process is. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. endothermic reaction examples.
from eduinput.com
for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. Determine if a chemical process is. revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total internal energy level or enthalpy. endothermic reaction examples. use bond dissociation energies to calculate enthalpy change or heat of reaction. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings.
Endothermic ReactionsCharacteristics, Identification, and Examples
Endothermic Reaction General Formula revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total internal energy level or enthalpy. Determine if a chemical process is. endothermic reaction examples. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. use bond dissociation energies to calculate enthalpy change or heat of reaction. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to.
From www.adda247.com
Endothermic Reaction Examples, Equations, Formula for Class 10 Endothermic Reaction General Formula revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released. Endothermic Reaction General Formula.
From www.savemyexams.com
Energy Diagrams College Board AP Chemistry Revision Notes 2022 Endothermic Reaction General Formula Determine if a chemical process is. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. use bond dissociation energies to calculate enthalpy change or heat of reaction. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. an. Endothermic Reaction General Formula.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID5403517 Endothermic Reaction General Formula When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. use bond dissociation energies to calculate enthalpy change or heat of reaction. an endothermic reaction is a chemical change in which. Endothermic Reaction General Formula.
From www.youtube.com
Chemical Reaction and equation (Exothermic and Endothermic Reaction Endothermic Reaction General Formula When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. use bond. Endothermic Reaction General Formula.
From dxosvaiso.blob.core.windows.net
Endothermic Reaction Of Respiration at Calvin Petillo blog Endothermic Reaction General Formula an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. use bond dissociation energies to calculate enthalpy change or heat of reaction. Determine if a chemical process is. an. Endothermic Reaction General Formula.
From lessonschoolallodial.z13.web.core.windows.net
Endothermic And Exothermic Reaction Diagram Endothermic Reaction General Formula an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. endothermic reaction examples. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. Endothermic Reaction General Formula.
From www.teachoo.com
Chemical Equation Meaning, How to Write [with 5+ Examples] Teachoo Endothermic Reaction General Formula an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total internal energy level or enthalpy. revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. for an endothermic chemical. Endothermic Reaction General Formula.
From www.slideserve.com
PPT Endothermic and Exothermic Reactions PowerPoint Presentation Endothermic Reaction General Formula endothermic reaction examples. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic. Endothermic Reaction General Formula.
From www.youtube.com
For an endothermic reaction, where `Delta H` represents the enthalpy of Endothermic Reaction General Formula an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. Determine if a chemical process is. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. use bond dissociation energies to calculate enthalpy change or heat of reaction. for an endothermic. Endothermic Reaction General Formula.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction General Formula revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. Determine if a chemical process is. use bond dissociation energies to. Endothermic Reaction General Formula.
From www.youtube.com
Endothermic & Exothermic reaction class 10 chemical reaction and Endothermic Reaction General Formula use bond dissociation energies to calculate enthalpy change or heat of reaction. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. endothermic reaction examples. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. When ammonium chloride (nh. Endothermic Reaction General Formula.
From www.vrogue.co
Endothermic Reaction Definition Examples Chemical Equ vrogue.co Endothermic Reaction General Formula an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total internal energy level or enthalpy. revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. an endothermic reaction is. Endothermic Reaction General Formula.
From guidelibcombusting.z13.web.core.windows.net
Energy Diagram Of Exothermic Reaction Endothermic Reaction General Formula use bond dissociation energies to calculate enthalpy change or heat of reaction. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total internal energy level or enthalpy. revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written. Endothermic Reaction General Formula.
From www.flexiprep.com
NCERT Class X Science Solutions Chapter 1 Chemical Reactions and Endothermic Reaction General Formula for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. endothermic reaction. Endothermic Reaction General Formula.
From www.onlinenotesnepal.com
Endothermic Reaction Thermodynamics in Grade 12 Chemistry Online Endothermic Reaction General Formula revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. Determine if a chemical process is. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. Endothermic Reaction General Formula.
From resolutionsforyou.com
The Journey of an Endothermic Reaction Understanding the Reaction Endothermic Reaction General Formula for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. use bond dissociation energies to calculate enthalpy change or heat of reaction. When ammonium chloride. Endothermic Reaction General Formula.
From www.numerade.com
SOLVED Distinguish between exothermic and endothermic reactions Endothermic Reaction General Formula use bond dissociation energies to calculate enthalpy change or heat of reaction. revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. endothermic reaction examples. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting. Endothermic Reaction General Formula.
From slideplayer.com
Chemistry Notes Chapter 2 ppt download Endothermic Reaction General Formula Determine if a chemical process is. endothermic reaction examples. revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. for an endothermic chemical reaction to proceed, the reactants must. Endothermic Reaction General Formula.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction General Formula Determine if a chemical process is. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total internal energy level or enthalpy. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. for an endothermic chemical reaction to proceed,. Endothermic Reaction General Formula.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction General Formula use bond dissociation energies to calculate enthalpy change or heat of reaction. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting. Endothermic Reaction General Formula.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Endothermic Reaction General Formula Determine if a chemical process is. revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. use bond dissociation energies to calculate enthalpy change or heat of reaction. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. for an endothermic. Endothermic Reaction General Formula.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction General Formula an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total internal energy level or enthalpy. use bond dissociation energies to calculate enthalpy change or heat of reaction. revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written. Endothermic Reaction General Formula.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction General Formula When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. an endothermic. Endothermic Reaction General Formula.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction General Formula use bond dissociation energies to calculate enthalpy change or heat of reaction. endothermic reaction examples. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total internal energy level or enthalpy. an endothermic reaction is a chemical reaction that absorbs thermal energy. Endothermic Reaction General Formula.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Endothermic Reaction General Formula When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. use bond dissociation energies to calculate enthalpy change or heat of reaction. endothermic reaction examples. an endothermic reaction is a chemical change in which the system absorbs thermal energy. Endothermic Reaction General Formula.
From asenoveo6schematic.z14.web.core.windows.net
How To Read Energy Diagrams Chemistry Endothermic Reaction General Formula revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. endothermic reaction examples. Determine if a chemical process is. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. an endothermic reaction is a. Endothermic Reaction General Formula.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID1846781 Endothermic Reaction General Formula for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. Determine if a chemical process is. endothermic reaction examples. use bond dissociation energies to. Endothermic Reaction General Formula.
From www.youtube.com
Endothermic or exothermic chemical equation YouTube Endothermic Reaction General Formula When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. revision notes on endothermic & exothermic reactions for the cambridge o level chemistry syllabus, written by the chemistry experts at save my exams. endothermic reaction examples. Determine if a chemical process is. an endothermic reaction is a chemical reaction that absorbs thermal energy from. Endothermic Reaction General Formula.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction General Formula Determine if a chemical process is. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. revision notes on endothermic & exothermic reactions for the. Endothermic Reaction General Formula.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction General Formula When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. Determine if a chemical process is. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. use bond dissociation energies to calculate enthalpy change or heat of reaction. revision notes on. Endothermic Reaction General Formula.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction General Formula an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total internal energy level or enthalpy. an endothermic reaction is. Endothermic Reaction General Formula.
From materialmediabrindle.z21.web.core.windows.net
Endothermic Reactions And Exothermic Reaction Endothermic Reaction General Formula an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. use bond dissociation energies to calculate enthalpy change or heat of reaction. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when. When ammonium chloride (nh 4 cl) is dissolved. Endothermic Reaction General Formula.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction General Formula an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total internal energy level or enthalpy. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. use bond dissociation energies to calculate enthalpy change or heat of reaction. Determine if a. Endothermic Reaction General Formula.
From www.youtube.com
Endothermic Reactions YouTube Endothermic Reaction General Formula When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total internal energy level or enthalpy. an endothermic reaction is a chemical reaction in which more energy is needed to break. Endothermic Reaction General Formula.
From mungfali.com
Endothermic Reaction Diagram Endothermic Reaction General Formula endothermic reaction examples. use bond dissociation energies to calculate enthalpy change or heat of reaction. Determine if a chemical process is. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. an endothermic reaction is a chemical reaction in which more energy is needed to break bonds. Endothermic Reaction General Formula.