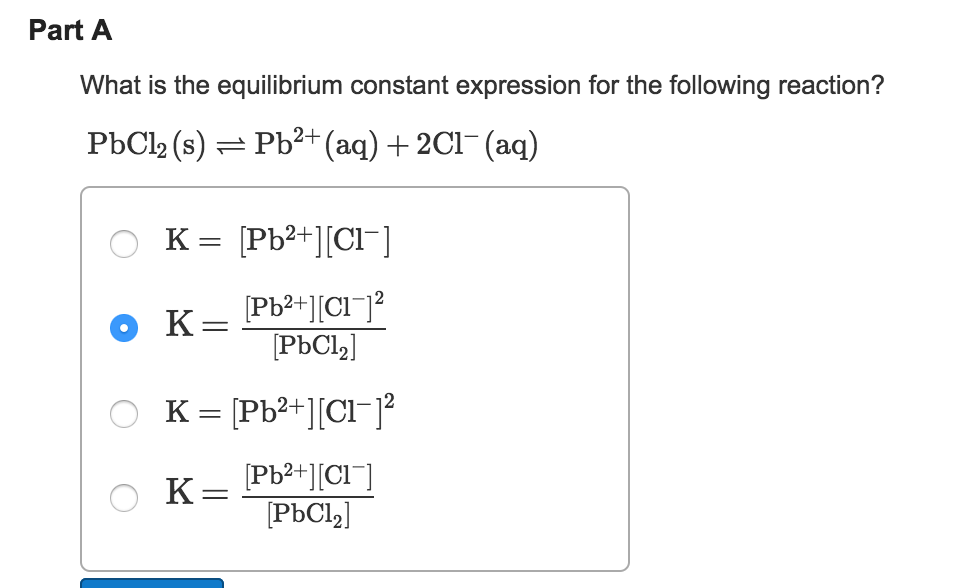
Solids And Liquids Are Not Included In The Equilibrium Constant Expression . Let me explain this with a different. Do not include the concentrations of pure solids and pure liquids in k eq. reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. the rule for heterogeneous equilibria is as follows: although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. [op] the equilibrium constant would not include the solid $\ce{i2}$, but why is this? a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can. when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant.
from learninglushers.z13.web.core.windows.net
Let me explain this with a different. the rule for heterogeneous equilibria is as follows: Do not include the concentrations of pure solids and pure liquids in k eq. a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can. [op] the equilibrium constant would not include the solid $\ce{i2}$, but why is this? reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these.
Equilibrium Constant Expression Worksheet
Solids And Liquids Are Not Included In The Equilibrium Constant Expression when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can. the rule for heterogeneous equilibria is as follows: Let me explain this with a different. when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. [op] the equilibrium constant would not include the solid $\ce{i2}$, but why is this? although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. Do not include the concentrations of pure solids and pure liquids in k eq.
From studylib.net
Equilibrium for a general reaction Solids And Liquids Are Not Included In The Equilibrium Constant Expression Let me explain this with a different. a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can. the rule for heterogeneous equilibria is as follows: Do not include the concentrations of pure solids and pure liquids in k eq. reactions containing pure solids and. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.youtube.com
Writing Equilibrium Expressions YouTube Solids And Liquids Are Not Included In The Equilibrium Constant Expression Do not include the concentrations of pure solids and pure liquids in k eq. the rule for heterogeneous equilibria is as follows: when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these.. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.slideserve.com
PPT Equilibrium Expressions PowerPoint Presentation, free download Solids And Liquids Are Not Included In The Equilibrium Constant Expression Let me explain this with a different. the rule for heterogeneous equilibria is as follows: Do not include the concentrations of pure solids and pure liquids in k eq. a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can. [op] the equilibrium constant would. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.youtube.com
Explain why pure liquids and solids can be ignored while writing the Solids And Liquids Are Not Included In The Equilibrium Constant Expression although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. the rule for heterogeneous equilibria is as follows: Let me explain this with a different. when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. reactions containing pure solids and liquids. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.slideserve.com
PPT Unit 9 and Equilibrium PowerPoint Presentation, free Solids And Liquids Are Not Included In The Equilibrium Constant Expression although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. [op] the equilibrium constant would not include the solid $\ce{i2}$, but why is this? Do not include the concentrations of pure solids and pure liquids in k eq. when dealing with these equilibria, remember that solids and pure liquids. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.coursehero.com
[Solved] 1. Write the equilibrium constant expressions for the Solids And Liquids Are Not Included In The Equilibrium Constant Expression the rule for heterogeneous equilibria is as follows: Let me explain this with a different. [op] the equilibrium constant would not include the solid $\ce{i2}$, but why is this? reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. although the activities of pure liquids or solids are. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.youtube.com
⚗️ Writing Equilibrium Expressions for Reactions Involving a Solid or a Solids And Liquids Are Not Included In The Equilibrium Constant Expression Do not include the concentrations of pure solids and pure liquids in k eq. when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. reactions containing pure solids and liquids results in. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From study.com
How to Write a Concentration Equilibrium Constant Expression Solids And Liquids Are Not Included In The Equilibrium Constant Expression the rule for heterogeneous equilibria is as follows: a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can. reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. Do not include the concentrations of pure solids. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.youtube.com
SolidLiquid Chemical Equilibrium YouTube Solids And Liquids Are Not Included In The Equilibrium Constant Expression when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. Let me explain this with a different. although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From study.com
How to Write Pressure Equilibrium Constant Expressions Chemistry Solids And Liquids Are Not Included In The Equilibrium Constant Expression the rule for heterogeneous equilibria is as follows: although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. Do not include the concentrations of pure solids and pure liquids in k eq. [op] the equilibrium constant would not include the solid $\ce{i2}$, but why is this? reactions containing. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.youtube.com
15.5 Heterogeneous Equilibrium Equilibrium Expression Involving a Solids And Liquids Are Not Included In The Equilibrium Constant Expression Do not include the concentrations of pure solids and pure liquids in k eq. when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. the rule for heterogeneous equilibria is as follows: [op] the equilibrium constant would not include the solid $\ce{i2}$, but why is this? Let me explain this. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.coursehero.com
[Solved] Write out the equilibrium constant expressions for the Solids And Liquids Are Not Included In The Equilibrium Constant Expression although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. a solid or. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From study.com
How to Write Equilibrium Expressions for Heterogeneous Reactions Solids And Liquids Are Not Included In The Equilibrium Constant Expression the rule for heterogeneous equilibria is as follows: when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. Do not include the concentrations of pure solids and pure liquids in k eq. Let me explain this with a different. although the activities of pure liquids or solids are not written. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID2224637 Solids And Liquids Are Not Included In The Equilibrium Constant Expression when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. the rule for heterogeneous equilibria is as follows: Let me explain this with a different. [op] the equilibrium constant would not. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From present5.com
Chapter 3 Chemical Equilibrium Reactions Irreversible Reversible The Solids And Liquids Are Not Included In The Equilibrium Constant Expression a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can. the rule for heterogeneous equilibria is as follows: when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. although the activities of pure liquids or solids. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From slideplayer.com
Ch. 13 Fundamental Equilibrium Concepts ppt download Solids And Liquids Are Not Included In The Equilibrium Constant Expression a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can. reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. [op] the equilibrium constant would not include the solid $\ce{i2}$, but why is this? Let me. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From slideplayer.com
Chapter 13 Equilibrium Dr. Walker DE Chemistry. ppt download Solids And Liquids Are Not Included In The Equilibrium Constant Expression a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can. Let me explain this with a different. Do not include the concentrations of pure solids and pure liquids in k eq. [op] the equilibrium constant would not include the solid $\ce{i2}$, but why is this?. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID Solids And Liquids Are Not Included In The Equilibrium Constant Expression Do not include the concentrations of pure solids and pure liquids in k eq. reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. Let me explain this with a different. . Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation Solids And Liquids Are Not Included In The Equilibrium Constant Expression [op] the equilibrium constant would not include the solid $\ce{i2}$, but why is this? the rule for heterogeneous equilibria is as follows: a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can. reactions containing pure solids and liquids results in heterogeneous reactions in. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From slideplayer.com
Chemical Equilibrium Chapter 13 4 out of 75 m/c Free Response Required Solids And Liquids Are Not Included In The Equilibrium Constant Expression reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. Do not include the. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.toppr.com
The concentration of a pure solid or liquid phase is not included in Solids And Liquids Are Not Included In The Equilibrium Constant Expression when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. Do not include the concentrations of pure solids and pure liquids in k eq. although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. a solid or liquid that should take part. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.numerade.com
SOLVEDIn a final equilibrium expression solids and liquid have value Solids And Liquids Are Not Included In The Equilibrium Constant Expression Let me explain this with a different. the rule for heterogeneous equilibria is as follows: reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. [op] the equilibrium constant would. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.slideserve.com
PPT Section 8.2—Equilibrium Constant PowerPoint Presentation, free Solids And Liquids Are Not Included In The Equilibrium Constant Expression [op] the equilibrium constant would not include the solid $\ce{i2}$, but why is this? when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. Let me explain this with a different. the rule for heterogeneous equilibria is as follows: a solid or liquid that should take part in a. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From learninglushers.z13.web.core.windows.net
Equilibrium Constant Expression Worksheet Solids And Liquids Are Not Included In The Equilibrium Constant Expression although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. Do not include the concentrations of pure solids and pure liquids in k eq. Let me explain this with a different. the. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.slideserve.com
PPT Solutions and Equilibrium PowerPoint Presentation, free download Solids And Liquids Are Not Included In The Equilibrium Constant Expression although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. the rule for heterogeneous equilibria is as follows: when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. reactions containing pure solids and liquids results in heterogeneous reactions in which the. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.numerade.com
SOLVED\( \mathrm{} \) \( \mathrm{} \) Solids And Liquids Are Not Included In The Equilibrium Constant Expression the rule for heterogeneous equilibria is as follows: a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can. when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. reactions containing pure solids and liquids results in. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.numerade.com
SOLVEDWrite an equilibrium expression for each chemical equation Solids And Liquids Are Not Included In The Equilibrium Constant Expression Do not include the concentrations of pure solids and pure liquids in k eq. a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can. although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. [op] the. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From learningchemistryeasily.blogspot.com
Learning Chemistry Easily Chemical Equilibrium Theory, An Introduction Solids And Liquids Are Not Included In The Equilibrium Constant Expression when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.chegg.com
Solved I. Which phases are not included in equilibrium Solids And Liquids Are Not Included In The Equilibrium Constant Expression when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.youtube.com
The equilibrium expression and the equilibrium constant (Keq) YouTube Solids And Liquids Are Not Included In The Equilibrium Constant Expression reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can. [op] the equilibrium constant would not include the solid $\ce{i2}$, but why is this? the. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.youtube.com
15.5 Heterogeneous Equilibria Reactions Involving a Solid or a Liquid Solids And Liquids Are Not Included In The Equilibrium Constant Expression a solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can. although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. the rule for heterogeneous equilibria is as follows: Do not include the concentrations of pure solids. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation Solids And Liquids Are Not Included In The Equilibrium Constant Expression the rule for heterogeneous equilibria is as follows: [op] the equilibrium constant would not include the solid $\ce{i2}$, but why is this? although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. Let me explain this with a different. Do not include the concentrations of pure solids and pure. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From slideplayer.com
Equilibrium. ppt download Solids And Liquids Are Not Included In The Equilibrium Constant Expression the rule for heterogeneous equilibria is as follows: Do not include the concentrations of pure solids and pure liquids in k eq. [op] the equilibrium constant would not include the solid $\ce{i2}$, but why is this? when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. a solid or. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.coursehero.com
[Solved] 1. Write the equilibrium constant expressions for the Solids And Liquids Are Not Included In The Equilibrium Constant Expression reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. when dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant. although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. Let me explain this. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.
From www.numerade.com
SOLVEDWhy are the concentrations of pure liquids and solids not Solids And Liquids Are Not Included In The Equilibrium Constant Expression although the activities of pure liquids or solids are not written explicitly in the equilibrium constant expression, these. the rule for heterogeneous equilibria is as follows: reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and. Do not include the concentrations of pure solids and pure liquids in k. Solids And Liquids Are Not Included In The Equilibrium Constant Expression.