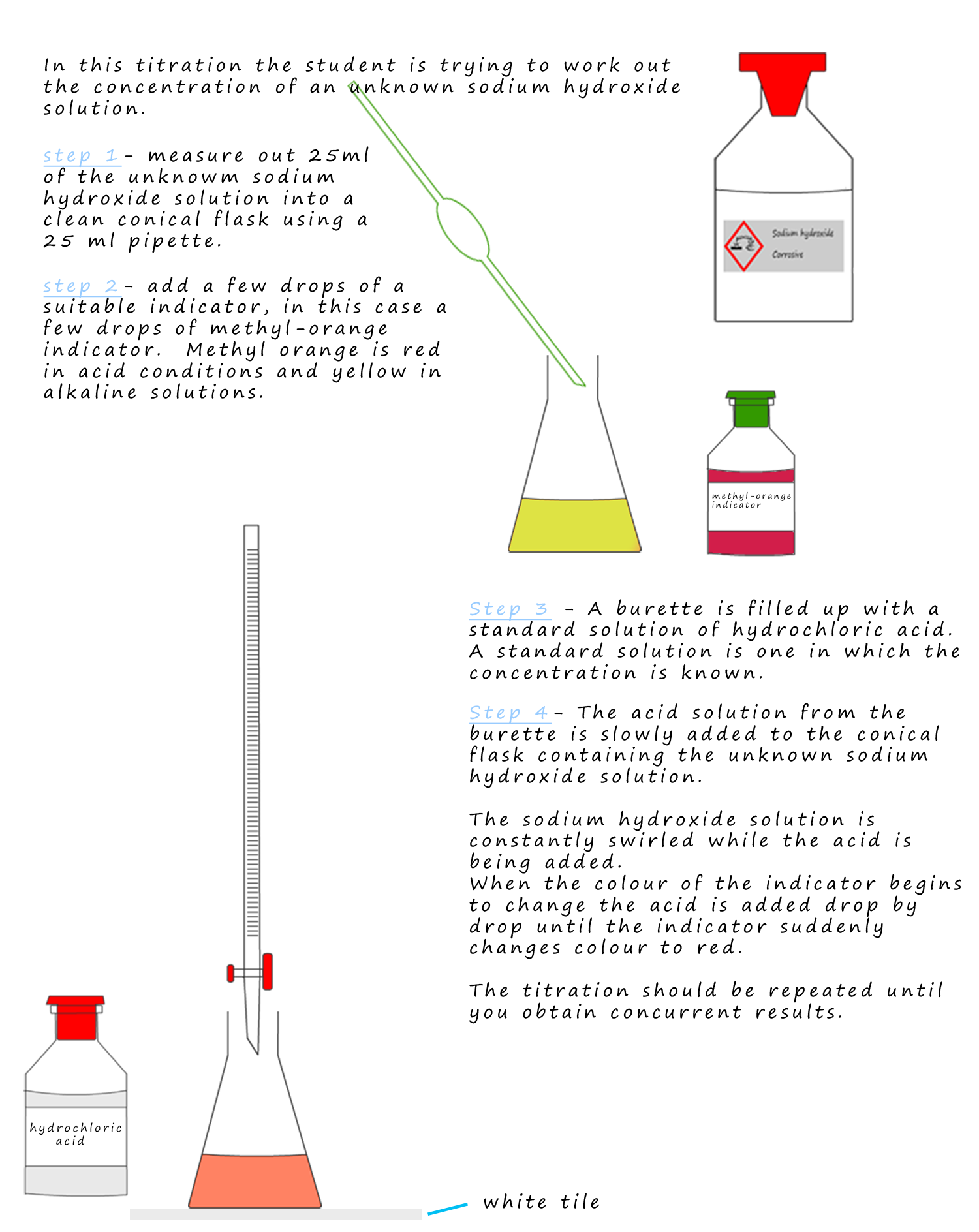
Titration Test Solution . By this process, the acid or. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Titration requires a solution of accurately known concentration called a standard solution. Such solutions should be so adjusted. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. If the unknown solution is basic then the standard. Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time.
from www.science-revision.co.uk
A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. By this process, the acid or. Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. Such solutions should be so adjusted. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. If the unknown solution is basic then the standard. Titration requires a solution of accurately known concentration called a standard solution.
Titrations
Titration Test Solution Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration requires a solution of accurately known concentration called a standard solution. By this process, the acid or. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Such solutions should be so adjusted. If the unknown solution is basic then the standard. Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Test Solution Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Such solutions should be so adjusted. If the unknown solution. Titration Test Solution.
From www.homesciencetools.com
Titration Kit Titration Lab Equipment for Chemistry HST Titration Test Solution By this process, the acid or. Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. If the unknown. Titration Test Solution.
From www.flinnsci.com
AcidBase Titrations—Classic Lab Kit for AP® Chemistry Flinn Scientific Titration Test Solution Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration requires a solution of accurately known concentration called a standard solution. If the unknown solution is basic then the standard. A titration is a technique used in chemistry to help determine the concentration. Titration Test Solution.
From chem.libretexts.org
9.3 Complexation Titrations Chemistry LibreTexts Titration Test Solution A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Such solutions should be so adjusted. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration requires a solution of accurately known. Titration Test Solution.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration Test Solution Titration requires a solution of accurately known concentration called a standard solution. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. If the unknown. Titration Test Solution.
From www.alamy.com
Titration experiment, adding chemical of known concentration to test Titration Test Solution Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Such solutions should be so adjusted. Titration requires a. Titration Test Solution.
From www.slideserve.com
PPT ENZYME CATALYSIS LAB PowerPoint Presentation, free download ID Titration Test Solution Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Titration requires a solution of accurately known concentration called a standard solution. Titration. Titration Test Solution.
From www.expresssolutions.us
Alkaline Titration Test Kit Express! Solutions Titration Test Solution Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration requires a solution of accurately. Titration Test Solution.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Test Solution Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. Titration requires a solution of accurately known concentration called a standard solution. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of. Titration Test Solution.
From www.alamy.com
Titration, titrimetry or volumetric analysis. A burette and Erlenmeyer Titration Test Solution Titration requires a solution of accurately known concentration called a standard solution. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. If the unknown solution is basic then the standard. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of. Titration Test Solution.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Test Solution Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. By this process, the. Titration Test Solution.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Test Solution Such solutions should be so adjusted. By this process, the acid or. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration requires a solution of accurately known concentration called a standard solution. A titration is a technique used in chemistry to help. Titration Test Solution.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Test Solution If the unknown solution is basic then the standard. By this process, the acid or. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration requires a solution of accurately known concentration called a standard solution. Titration (also known as titrimetry[ 1 ] and volumetric. Titration Test Solution.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Test Solution A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Such solutions should be so adjusted. By this process, the acid or. If the unknown solution is basic then the standard. Titration requires a solution of accurately known concentration called a standard solution. Titration is a quantitative analysis to. Titration Test Solution.
From www.ofite.com
OFI Testing Equipment, Inc. Total Hardness Titration Kit Titration Test Solution Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. By this process, the acid or. Such solutions should be so adjusted. Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. Titration Test Solution.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Test Solution Such solutions should be so adjusted. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration requires a solution of accurately known concentration called a standard solution. Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of. Titration Test Solution.
From www.birmingham.ac.uk
Chemistry titrations University of Birmingham Titration Test Solution Titration requires a solution of accurately known concentration called a standard solution. By this process, the acid or. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical. Titration Test Solution.
From www.electro-glo.com
Potassium IodideIodate Titrating Solution ElectroGlo Distribution Titration Test Solution Such solutions should be so adjusted. Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at. Titration Test Solution.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Test Solution Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Titration is a quantitative analysis to determine the concentration. Titration Test Solution.
From www.youtube.com
Total Water Hardness using EDTA Titration YouTube Titration Test Solution Such solutions should be so adjusted. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration requires a solution of accurately known concentration called a standard solution. By this process, the acid or. Titration is a quantitative analysis to determine the concentration of an unknown. Titration Test Solution.
From www.sliderbase.com
Titration Titration Test Solution Titration requires a solution of accurately known concentration called a standard solution. Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known. Titration Test Solution.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Titration Test Solution Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration requires a solution of accurately known concentration called a standard solution. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Titration. Titration Test Solution.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Test Solution Titration requires a solution of accurately known concentration called a standard solution. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Such solutions should be so adjusted. By this process, the acid or. If the unknown solution is basic then the standard. Titration (also known as titrimetry[ 1. Titration Test Solution.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Titration Test Solution Titration requires a solution of accurately known concentration called a standard solution. If the unknown solution is basic then the standard. Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. Titration is the slow addition of one solution of. Titration Test Solution.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Titration Test Solution By this process, the acid or. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. Such solutions should. Titration Test Solution.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Test Solution Titration requires a solution of accurately known concentration called a standard solution. If the unknown solution is basic then the standard. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. By this process, the acid or. Such solutions should be so adjusted. Titration (also known as titrimetry[ 1. Titration Test Solution.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Test Solution Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. By this process, the acid or. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. If the unknown solution. Titration Test Solution.
From www.youtube.com
Titration of Mohr Salt Solution with Standard Potassium Dichromate Titration Test Solution Such solutions should be so adjusted. By this process, the acid or. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. Titration Test Solution.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Test Solution If the unknown solution is basic then the standard. Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. Titration Test Solution.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Test Solution A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. Such solutions should be so adjusted. If the unknown. Titration Test Solution.
From www.electro-glo.com
Ceric Sulfate Titrating Solution, Eyedropper Dispenser ElectroGlo Titration Test Solution Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration requires a solution of accurately known concentration called a standard solution. Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. Titration Test Solution.
From www.science-revision.co.uk
Titrations Titration Test Solution Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. Such solutions should be so adjusted. If the unknown solution is basic then the standard. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding. Titration Test Solution.
From uwaterloo.ca
Classic acid and base titration in pink Chem 13 News Magazine Titration Test Solution Titration requires a solution of accurately known concentration called a standard solution. Such solutions should be so adjusted. If the unknown solution is basic then the standard. By this process, the acid or. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Titration is a quantitative analysis to. Titration Test Solution.
From www.logicoil.com
TAN TBN Titrator LogicOil Titration Test Solution Such solutions should be so adjusted. Titration requires a solution of accurately known concentration called a standard solution. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. If the unknown solution is basic then the standard. Titration is the slow addition of one. Titration Test Solution.
From mirjamglessmer.com
Measuring the concentration of dissolved oxygen in sea water Part 3 Titration Test Solution Titration (also known as titrimetry[ 1 ] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to. Such solutions should be so adjusted. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. Titration is the. Titration Test Solution.