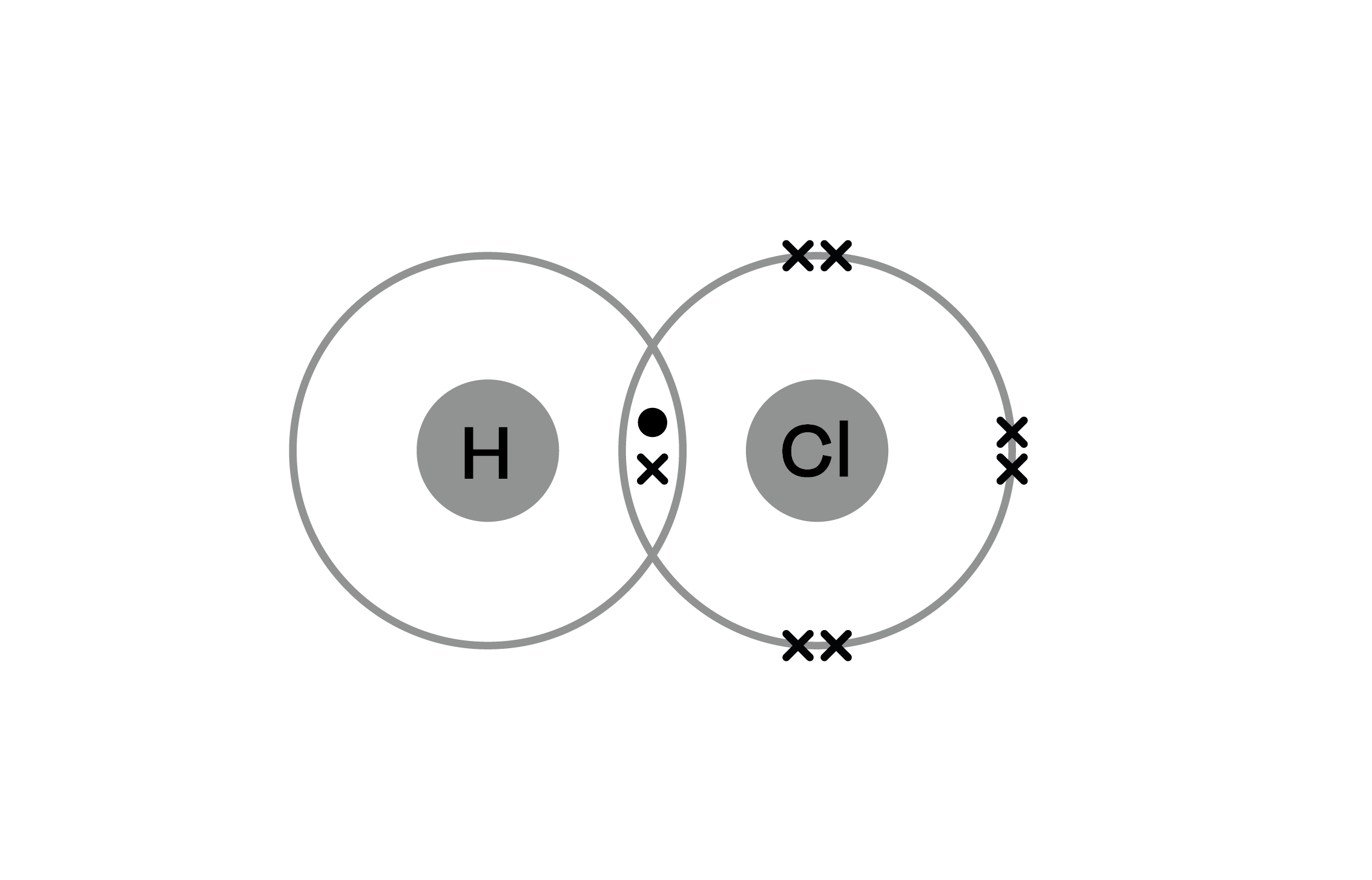
Bromine Dioxide Covalent . These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Covalent bonding is an important and extensive concept in. Ionic compounds generally form from metals and nonmetals. Bromine compounds are compounds containing the element bromine (br). Compounds that do not contain ions, but instead consist of atoms bonded tightly. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were.
from evulpo.com
Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Ionic compounds generally form from metals and nonmetals. These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Bromine compounds are compounds containing the element bromine (br). Covalent bonding is an important and extensive concept in. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Compounds that do not contain ions, but instead consist of atoms bonded tightly.
Covalent bonding Chemistry Explanation & Exercises evulpo
Bromine Dioxide Covalent Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Covalent bonding is an important and extensive concept in. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Ionic compounds generally form from metals and nonmetals. Bromine compounds are compounds containing the element bromine (br).
From www.numerade.com
SOLVED The picture below represents the particle of a compound The Bromine Dioxide Covalent Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Bromine compounds are compounds containing the element bromine (br). Ionic compounds generally form from metals and nonmetals. Covalent bonding is an important and extensive concept in. These molecular compounds (covalent compounds) result when atoms share, rather than transfer. Bromine Dioxide Covalent.
From www.shutterstock.com
Double Covalent Bond Carbon Dioxide Molecule Stock Vector (Royalty Free Bromine Dioxide Covalent These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Bromine compounds are compounds containing the element bromine (br). Compounds that do not contain ions, but instead consist of atoms bonded tightly. Ionic compounds generally form from metals and nonmetals. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that. Bromine Dioxide Covalent.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Dioxide Covalent These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Bromine compounds are compounds containing the element bromine (br). Yes, br2 (bromine gas) is a molecule made up of two bromine atoms. Bromine Dioxide Covalent.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.46 Understand How to Use DotandCross Bromine Dioxide Covalent Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Covalent bonding is an important and extensive concept in. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Bromine compounds are compounds containing the element bromine (br). These molecular compounds (covalent compounds) result when. Bromine Dioxide Covalent.
From courses.lumenlearning.com
Chemical Bonds Anatomy and Physiology I Bromine Dioxide Covalent Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Covalent bonding is an important and extensive concept in. Ionic compounds generally form from metals and nonmetals. These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Bromine compounds are compounds containing. Bromine Dioxide Covalent.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.46 Understand How to Use DotandCross Bromine Dioxide Covalent Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Covalent bonding is an. Bromine Dioxide Covalent.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Bromine Dioxide Covalent These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Ionic compounds generally form from metals and nonmetals. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Bromine compounds are compounds containing the element bromine (br). Compounds that do not contain. Bromine Dioxide Covalent.
From facts.net
13 Unbelievable Facts About Covalent Bond Bromine Dioxide Covalent Covalent bonding is an important and extensive concept in. Ionic compounds generally form from metals and nonmetals. Bromine compounds are compounds containing the element bromine (br). Compounds that do not contain ions, but instead consist of atoms bonded tightly. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they. Bromine Dioxide Covalent.
From www.youtube.com
Covalent Bonding DotCross Diagrams GCSE Chemistry Revision YouTube Bromine Dioxide Covalent Covalent bonding is an important and extensive concept in. Bromine compounds are compounds containing the element bromine (br). Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they. Bromine Dioxide Covalent.
From www.linstitute.net
AQA A Level Chemistry复习笔记1.4.3 Covalent Bonding翰林国际教育 Bromine Dioxide Covalent Compounds that do not contain ions, but instead consist of atoms bonded tightly. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Ionic compounds generally form from metals and nonmetals. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of. Bromine Dioxide Covalent.
From geniebook.com
Covalent Bonding Secondary 3 Chemistry Geniebook Bromine Dioxide Covalent Bromine compounds are compounds containing the element bromine (br). Ionic compounds generally form from metals and nonmetals. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Covalent bonding is an important and. Bromine Dioxide Covalent.
From ar.inspiredpencil.com
Carbon Dioxide Covalent Bond Bromine Dioxide Covalent Covalent bonding is an important and extensive concept in. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Bromine compounds are compounds containing the element bromine (br). Ionic compounds generally form from metals and nonmetals. These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Yes, br2 (bromine gas) is. Bromine Dioxide Covalent.
From www.science-revision.co.uk
Covalent bonding Bromine Dioxide Covalent Covalent bonding is an important and extensive concept in. Bromine compounds are compounds containing the element bromine (br). Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent. Bromine Dioxide Covalent.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Dioxide Covalent Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Covalent bonding is an important and extensive concept in. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Compounds that do not contain ions, but instead. Bromine Dioxide Covalent.
From www.alamy.com
Covalent bond types, illustration Stock Photo Alamy Bromine Dioxide Covalent Ionic compounds generally form from metals and nonmetals. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Compounds that do not contain ions, but instead consist. Bromine Dioxide Covalent.
From evulpo.com
Covalent bonding Chemistry Explanation & Exercises evulpo Bromine Dioxide Covalent Covalent bonding is an important and extensive concept in. Bromine compounds are compounds containing the element bromine (br). Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Ionic compounds generally form from. Bromine Dioxide Covalent.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.46 Understand How to Use DotandCross Bromine Dioxide Covalent Covalent bonding is an important and extensive concept in. Bromine compounds are compounds containing the element bromine (br). Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Polyatomic ions are covalent units (molecules) where the. Bromine Dioxide Covalent.
From courses.lumenlearning.com
Covalent Bonds Biology for Majors I Bromine Dioxide Covalent These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Compounds. Bromine Dioxide Covalent.
From www.alamy.com
Bromine molecule Br2 Stock Photo Alamy Bromine Dioxide Covalent Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Bromine compounds are compounds containing the element bromine (br). Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Compounds that do not contain ions, but instead. Bromine Dioxide Covalent.
From www.alamy.com
Bromine monochloride hires stock photography and images Alamy Bromine Dioxide Covalent Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Ionic compounds generally form from metals and nonmetals. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that. Bromine Dioxide Covalent.
From www.chemistrylearner.com
Nonpolar Covalent Bond Definition and Examples Bromine Dioxide Covalent Compounds that do not contain ions, but instead consist of atoms bonded tightly. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Bromine compounds are compounds containing the element bromine (br). Covalent bonding is an important and extensive concept in. Ionic compounds generally form from metals and. Bromine Dioxide Covalent.
From socratic.org
What's the difference between a formula unit and a molecule? Socratic Bromine Dioxide Covalent Ionic compounds generally form from metals and nonmetals. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Covalent bonding is an important and extensive concept in.. Bromine Dioxide Covalent.
From www.thaipng.com
โบรมีน Dioxide, โบรมีน, Bromate png png โบรมีน Dioxide, โบรมีน Bromine Dioxide Covalent These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Bromine compounds are compounds containing the element bromine (br). Covalent bonding is an important and extensive concept in. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Compounds that do not contain. Bromine Dioxide Covalent.
From www.anyrgb.com
Pblock, homonuclear Molecule, bromine Dioxide, iodine127, diatomic Bromine Dioxide Covalent Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Ionic compounds generally form. Bromine Dioxide Covalent.
From definitionklw.blogspot.com
Double Covalent Bond Definition DEFINITION KLW Bromine Dioxide Covalent Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Compounds that do not contain ions, but instead consist of atoms bonded tightly. These molecular compounds (covalent. Bromine Dioxide Covalent.
From www.alamy.com
Covalent Radius The covalent radius is half of the distance between Bromine Dioxide Covalent These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Bromine compounds are compounds containing the element bromine (br).. Bromine Dioxide Covalent.
From www.numerade.com
Bromine is a halogen; one of the elements in the same column of the Bromine Dioxide Covalent Bromine compounds are compounds containing the element bromine (br). Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Ionic compounds generally form from metals and nonmetals. Yes, br2 (bromine gas) is a molecule made. Bromine Dioxide Covalent.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.46 Understand How to Use DotandCross Bromine Dioxide Covalent Ionic compounds generally form from metals and nonmetals. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Bromine compounds are compounds containing the element bromine (br). These molecular compounds (covalent compounds) result when atoms. Bromine Dioxide Covalent.
From 2012books.lardbucket.org
Polar Covalent Bonds Bromine Dioxide Covalent Covalent bonding is an important and extensive concept in. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Ionic compounds generally form from metals and nonmetals. Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Bromine compounds are compounds containing the element bromine. Bromine Dioxide Covalent.
From mungfali.com
Covalent Bond Of Carbon Dioxide Bromine Dioxide Covalent Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Bromine compounds are compounds. Bromine Dioxide Covalent.
From www.thesciencehive.co.uk
Covalent bonding (GCSE) — the science sauce Bromine Dioxide Covalent These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Covalent bonding is an important and extensive concept in. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Polyatomic. Bromine Dioxide Covalent.
From www.dreamstime.com
Covalent Bonds Including Single, Double, and Triple Bonds in Water Bromine Dioxide Covalent Ionic compounds generally form from metals and nonmetals. These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Bromine compounds are compounds containing the element bromine (br). Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Covalent bonding is an important and. Bromine Dioxide Covalent.
From mavink.com
Hydrogen Covalent Bond Diagram Bromine Dioxide Covalent Covalent bonding is an important and extensive concept in. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Bromine compounds are compounds containing the element bromine (br). Ionic compounds generally form from metals and nonmetals.. Bromine Dioxide Covalent.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen Bromine Dioxide Covalent Covalent bonding is an important and extensive concept in. These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Bromine compounds are compounds containing the element bromine (br). Yes, br2 (bromine gas) is a molecule made up of two bromine atoms that are bonded together through a covalent bond. Ionic compounds generally form from. Bromine Dioxide Covalent.
From www.showme.com
Sulfur Bromine Covalent Bonding Science ShowMe Bromine Dioxide Covalent Bromine compounds are compounds containing the element bromine (br). Polyatomic ions are covalent units (molecules) where the total number of protons \(\neq\) to the total number of electrons and they were. These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Yes, br2 (bromine gas) is a molecule made up of two bromine atoms. Bromine Dioxide Covalent.