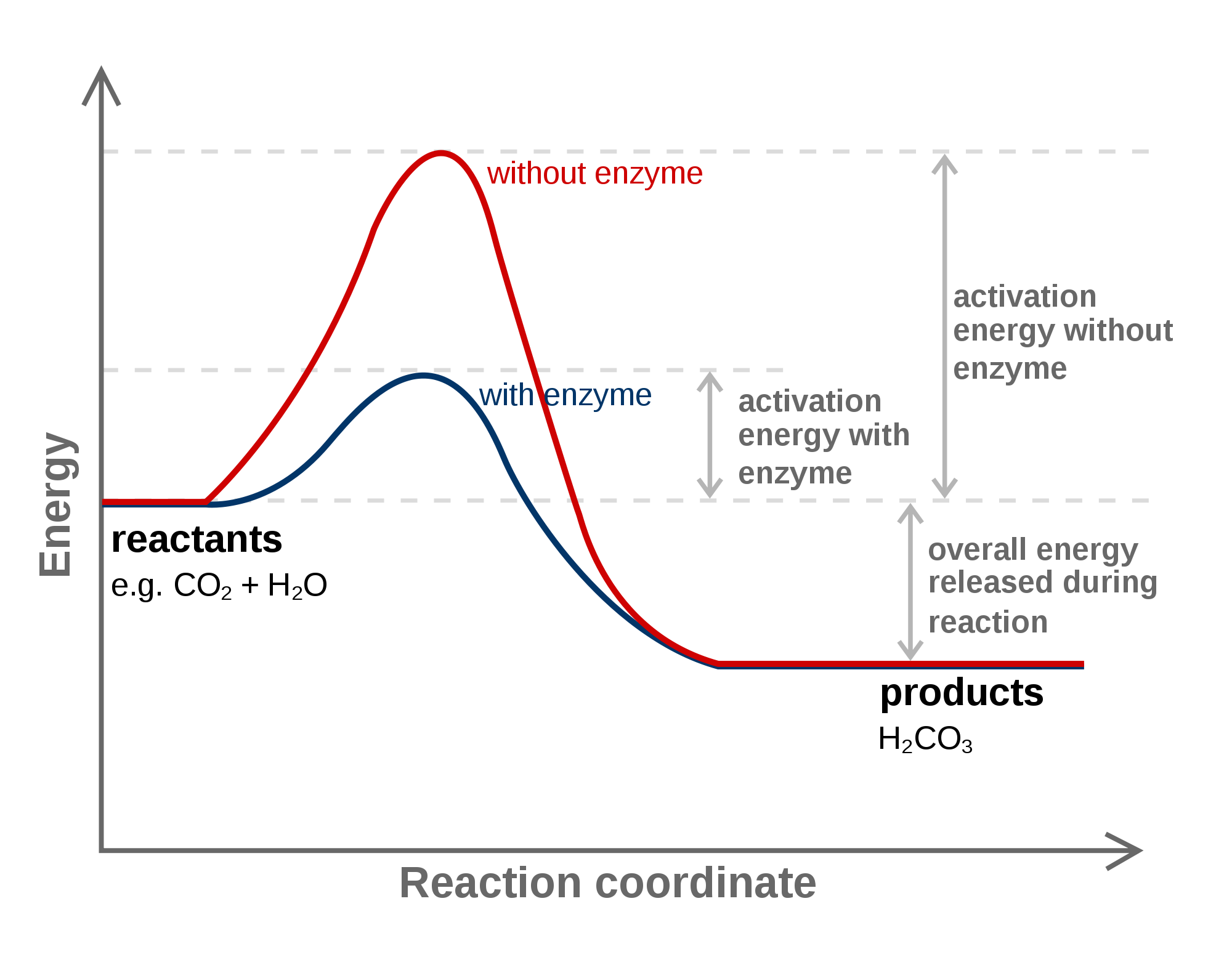
Catalyst Graph . a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts can be homogenous (in the same. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. this page explains how adding a catalyst affects the rate of a reaction. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within. List examples of catalysis in natural and industrial. It assumes familiarity with basic concepts in the collision theory of. The only effect of the catalyst is to lower.
from schematicbraginamh.z4.web.core.windows.net
It assumes familiarity with basic concepts in the collision theory of. List examples of catalysis in natural and industrial. a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. The only effect of the catalyst is to lower. Catalysts can be homogenous (in the same. this page explains how adding a catalyst affects the rate of a reaction.
Energy Diagram For Chemical Reaction
Catalyst Graph explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. List examples of catalysis in natural and industrial. The only effect of the catalyst is to lower. a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. this page explains how adding a catalyst affects the rate of a reaction. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Catalysts can be homogenous (in the same. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within. It assumes familiarity with basic concepts in the collision theory of.
From
Catalyst Graph The only effect of the catalyst is to lower. Catalysts can be homogenous (in the same. It assumes familiarity with basic concepts in the collision theory of. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface. Catalyst Graph.
From
Catalyst Graph gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within. this page explains how adding a catalyst affects the rate of a reaction. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. List examples of catalysis in natural and industrial. The only. Catalyst Graph.
From
Catalyst Graph this page explains how adding a catalyst affects the rate of a reaction. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts can be homogenous (in the same. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within. List examples of. Catalyst Graph.
From
Catalyst Graph Catalysts can be homogenous (in the same. List examples of catalysis in natural and industrial. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst is a substance which speeds up a. Catalyst Graph.
From www.chegg.com
Solved The Diagram Shown Above Shows The Reaction Profile... Catalyst Graph Catalysts can be homogenous (in the same. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. The only. Catalyst Graph.
From
Catalyst Graph Catalysts can be homogenous (in the same. List examples of catalysis in natural and industrial. a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. It assumes familiarity with basic concepts in the collision theory of. explain the function of a catalyst in terms of reaction mechanisms and. Catalyst Graph.
From
Catalyst Graph gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within. Catalysts can be homogenous (in the same. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. The only effect of the catalyst is to lower. It assumes familiarity with basic concepts in the. Catalyst Graph.
From
Catalyst Graph The only effect of the catalyst is to lower. It assumes familiarity with basic concepts in the collision theory of. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within. this page explains how adding a catalyst affects the rate of a reaction. explain the function of a. Catalyst Graph.
From www.radleys.com
Case Studies in Catalysis Success Stories and Tips for More Efficient Catalyst Graph explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. List examples of catalysis in natural and industrial. a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the. Catalyst Graph.
From
Catalyst Graph List examples of catalysis in natural and industrial. The only effect of the catalyst is to lower. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. catalysts affect the rate of a chemical reaction by altering. Catalyst Graph.
From
Catalyst Graph Catalysts can be homogenous (in the same. It assumes familiarity with basic concepts in the collision theory of. this page explains how adding a catalyst affects the rate of a reaction. The only effect of the catalyst is to lower. List examples of catalysis in natural and industrial. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur. Catalyst Graph.
From chemistry.stackexchange.com
physical chemistry Which diagram shows the effect of catalysis on Catalyst Graph learn how catalysts speed up reactions by providing an alternative route with lower activation energy. It assumes familiarity with basic concepts in the collision theory of. a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. Catalysts can be homogenous (in the same. this page explains how. Catalyst Graph.
From
Catalyst Graph learn how catalysts speed up reactions by providing an alternative route with lower activation energy. a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. this page explains how adding a catalyst affects the rate of a reaction. explain the function of a catalyst in terms. Catalyst Graph.
From
Catalyst Graph Catalysts can be homogenous (in the same. this page explains how adding a catalyst affects the rate of a reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It assumes familiarity with basic concepts in the collision theory of. List examples of catalysis in natural and industrial. . Catalyst Graph.
From
Catalyst Graph learn how catalysts speed up reactions by providing an alternative route with lower activation energy. List examples of catalysis in natural and industrial. a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. It assumes familiarity with basic concepts in the collision theory of. explain the function. Catalyst Graph.
From
Catalyst Graph It assumes familiarity with basic concepts in the collision theory of. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. The only effect of the catalyst is to lower. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in. Catalyst Graph.
From
Catalyst Graph catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. List examples of catalysis in natural and industrial. a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. It assumes familiarity with basic concepts in the collision theory of. . Catalyst Graph.
From
Catalyst Graph The only effect of the catalyst is to lower. this page explains how adding a catalyst affects the rate of a reaction. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction.. Catalyst Graph.
From
Catalyst Graph It assumes familiarity with basic concepts in the collision theory of. Catalysts can be homogenous (in the same. List examples of catalysis in natural and industrial. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation. Catalyst Graph.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst Graph Catalysts can be homogenous (in the same. It assumes familiarity with basic concepts in the collision theory of. a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. catalysts affect the rate. Catalyst Graph.
From
Catalyst Graph a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. It assumes familiarity with. Catalyst Graph.
From
Catalyst Graph gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst is a. Catalyst Graph.
From
Catalyst Graph a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. The only effect of the catalyst is to lower. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. explain the function of a catalyst in terms of reaction mechanisms and potential. Catalyst Graph.
From psu.pb.unizin.org
12.2 Factors Affecting Reaction Rates (2018) Chemistry 112 Chapters Catalyst Graph learn how catalysts speed up reactions by providing an alternative route with lower activation energy. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts can be homogenous (in the same. gas. Catalyst Graph.
From physics.stackexchange.com
material science What properties make a good catalyst? Physics Catalyst Graph a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. List examples of catalysis in natural and industrial. The only effect of the catalyst is to lower. this page explains how adding. Catalyst Graph.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Catalyst Graph List examples of catalysis in natural and industrial. It assumes familiarity with basic concepts in the collision theory of. Catalysts can be homogenous (in the same. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. explain the function of a catalyst in terms of reaction mechanisms and potential energy. Catalyst Graph.
From
Catalyst Graph The only effect of the catalyst is to lower. a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. this page explains how adding a catalyst affects the rate of a reaction.. Catalyst Graph.
From
Catalyst Graph learn how catalysts speed up reactions by providing an alternative route with lower activation energy. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same. a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end. Catalyst Graph.
From
Catalyst Graph The only effect of the catalyst is to lower. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Catalysts can be homogenous (in the same. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within. catalysts affect the rate of a chemical. Catalyst Graph.
From
Catalyst Graph a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. List examples of catalysis in natural and industrial. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. learn how catalysts speed up reactions by providing an alternative route with lower activation. Catalyst Graph.
From infinitylearn.com
A catalyst increases the rate of reaction by Sri Chaitanya Infinity Catalyst Graph a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. catalysts affect the rate of a. Catalyst Graph.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Catalyst Graph this page explains how adding a catalyst affects the rate of a reaction. Catalysts can be homogenous (in the same. List examples of catalysis in natural and industrial. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of. Catalyst Graph.
From
Catalyst Graph explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within. Catalysts can be homogenous (in the same. . Catalyst Graph.
From schematicbraginamh.z4.web.core.windows.net
Energy Diagram For Chemical Reaction Catalyst Graph this page explains how adding a catalyst affects the rate of a reaction. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within. Catalysts can be homogenous (in the same. a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of. Catalyst Graph.
From www.sarthaks.com
The graph of the effect of catalyst on activation energy is given below Catalyst Graph gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within. It assumes familiarity with basic concepts in the collision theory of. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. List examples of catalysis in natural and industrial. catalysts affect the rate. Catalyst Graph.