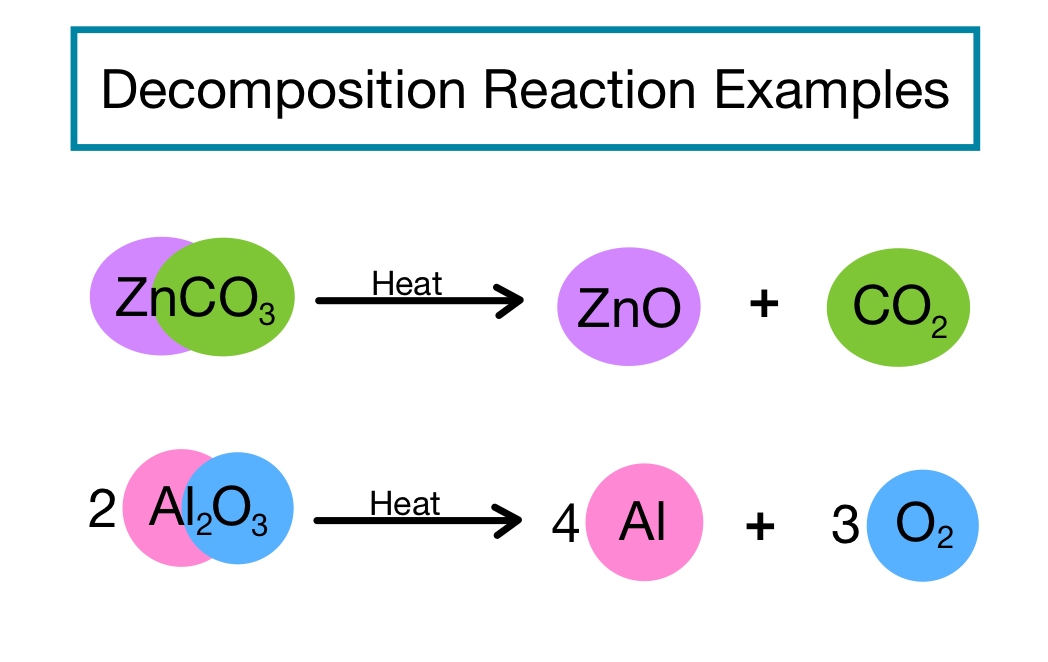
Catalyst Decomposition Example . Includes kit list and safety instructions. See how to test catalysts using hydrogen peroxide decomposition and. Enzyme inhibitors cause a decrease in the reaction rate of. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. A classic example of this is an. H 2 co 3 (aq.) → co 2 (g) + h 2 o (l) A homogeneous catalyst is present in the same phase as the reactants. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2]. It interacts with a reactant to form an intermediate substance, which then. One single reactant and two or more products; Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst.
from www.expii.com
Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. See how to test catalysts using hydrogen peroxide decomposition and. H 2 co 3 (aq.) → co 2 (g) + h 2 o (l) One single reactant and two or more products; Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. A homogeneous catalyst is present in the same phase as the reactants. An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2]. A classic example of this is an. Enzyme inhibitors cause a decrease in the reaction rate of. Includes kit list and safety instructions.
Reactions — Definition & Examples Expii
Catalyst Decomposition Example A classic example of this is an. A homogeneous catalyst is present in the same phase as the reactants. One single reactant and two or more products; See how to test catalysts using hydrogen peroxide decomposition and. It interacts with a reactant to form an intermediate substance, which then. Includes kit list and safety instructions. H 2 co 3 (aq.) → co 2 (g) + h 2 o (l) Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. A classic example of this is an. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Enzyme inhibitors cause a decrease in the reaction rate of. Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2].
From www.vrogue.co
Reaction Characteristics vrogue.co Catalyst Decomposition Example Includes kit list and safety instructions. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2]. A homogeneous catalyst is present in the. Catalyst Decomposition Example.
From www.expii.com
Reactions — Definition & Examples Expii Catalyst Decomposition Example Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. A homogeneous catalyst is present in the same phase as the reactants. A classic example of this is an. An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o). Catalyst Decomposition Example.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Decomposition Example Includes kit list and safety instructions. It interacts with a reactant to form an intermediate substance, which then. Enzyme inhibitors cause a decrease in the reaction rate of. H 2 co 3 (aq.) → co 2 (g) + h 2 o (l) One single reactant and two or more products; Catalytic decomposition is when a decomposition reaction occurs with the. Catalyst Decomposition Example.
From www.pnas.org
Active sites and mechanisms for H2O2 over Pd catalysts PNAS Catalyst Decomposition Example An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2]. H 2 co 3 (aq.) → co 2 (g) + h 2 o (l) Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. A classic example of. Catalyst Decomposition Example.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Catalyst Decomposition Example Includes kit list and safety instructions. It interacts with a reactant to form an intermediate substance, which then. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Enzyme inhibitors cause a decrease in the reaction rate of. One single reactant and two or more products; H 2 co 3 (aq.) → co 2 (g). Catalyst Decomposition Example.
From brealchandlerphysicalscience.weebly.com
Catalyst Decomposition Example Includes kit list and safety instructions. H 2 co 3 (aq.) → co 2 (g) + h 2 o (l) An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2]. A homogeneous catalyst is present in the same phase as the reactants. A. Catalyst Decomposition Example.
From www.scribd.com
Catalysts for the of Hydrogen Peroxide Catalysis Catalyst Decomposition Example H 2 co 3 (aq.) → co 2 (g) + h 2 o (l) Enzyme inhibitors cause a decrease in the reaction rate of. Includes kit list and safety instructions. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. One single reactant and two or more products; Catalytic decomposition is when a decomposition reaction. Catalyst Decomposition Example.
From www.researchgate.net
Possible pathway for catalyst (top) and the pKa values of Catalyst Decomposition Example Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Includes kit list and safety instructions. One single reactant and two or more products; Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. A classic example of this is an. See how to test. Catalyst Decomposition Example.
From www.slideserve.com
PPT CATALYSIS BY ACIDS AND BASES PowerPoint Presentation, free Catalyst Decomposition Example Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Includes kit list and safety instructions. Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. One single reactant and two. Catalyst Decomposition Example.
From classfullturfites.z13.web.core.windows.net
Examples Of Reactions Chemistry Catalyst Decomposition Example An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2]. See how to test catalysts using hydrogen peroxide decomposition and. Includes kit list and safety instructions. Enzyme inhibitors cause a decrease in the reaction rate of. A homogeneous catalyst is present in the. Catalyst Decomposition Example.
From byjus.com
of H2O2 is catalysed by (1) H3PO4 (2) Acetamilide (3) HF Catalyst Decomposition Example Includes kit list and safety instructions. One single reactant and two or more products; It interacts with a reactant to form an intermediate substance, which then. An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2]. Catalytic decomposition is when a decomposition reaction. Catalyst Decomposition Example.
From slideplayer.com
Chemical Reactions. ppt download Catalyst Decomposition Example Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. A classic example of this is an. H 2 co 3 (aq.) → co 2 (g) + h 2 o (l) Includes kit list and safety instructions. One single reactant and two or more products; See how to test catalysts using hydrogen peroxide decomposition and.. Catalyst Decomposition Example.
From chemicaltweak.com
Types Of Reaction Definition With Examples Equations Catalyst Decomposition Example A classic example of this is an. Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Enzyme inhibitors cause a decrease in the reaction rate of. H 2 co 3 (aq.) → co 2 (g) + h 2 o (l). Catalyst Decomposition Example.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation Catalyst Decomposition Example Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. See how to test catalysts using hydrogen peroxide decomposition and. H 2 co 3 (aq.) → co 2 (g) + h 2 o (l) One single reactant and two or more. Catalyst Decomposition Example.
From www.tes.com
reactions KS3 Activate Science Teaching Resources Catalyst Decomposition Example Includes kit list and safety instructions. A classic example of this is an. Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. See how to test catalysts using hydrogen peroxide decomposition and. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. A homogeneous catalyst. Catalyst Decomposition Example.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Catalyst Decomposition Example H 2 co 3 (aq.) → co 2 (g) + h 2 o (l) An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2]. A homogeneous catalyst is present in the same phase as the reactants. Enzyme inhibitors cause a decrease in the. Catalyst Decomposition Example.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalyst Decomposition Example Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. Includes kit list and safety instructions. See how to test catalysts using hydrogen peroxide decomposition and. One single reactant and two or more products; Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. It interacts. Catalyst Decomposition Example.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts Catalyst Decomposition Example Enzyme inhibitors cause a decrease in the reaction rate of. Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. See how to test catalysts using hydrogen peroxide decomposition and. Collect a range of catalysts to explore the decomposition of hydrogen. Catalyst Decomposition Example.
From www.chemistrylearner.com
Reaction Definition, Examples, & Applications Catalyst Decomposition Example An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2]. H 2 co 3 (aq.) → co 2 (g) + h 2 o (l) A homogeneous catalyst is present in the same phase as the reactants. One single reactant and two or more. Catalyst Decomposition Example.
From www.vrogue.co
The Catalytic Of Hydrogen Peroxide Writ vrogue.co Catalyst Decomposition Example A homogeneous catalyst is present in the same phase as the reactants. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. One single reactant and two or more products; An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2. Catalyst Decomposition Example.
From www.mdpi.com
Catalysts Free FullText Advances in Catalytic of N2O Catalyst Decomposition Example Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. It interacts with a reactant to form an intermediate substance, which then. A homogeneous catalyst is present in the same phase as the reactants. Enzyme inhibitors cause a decrease in the reaction rate of. See how to test catalysts using hydrogen peroxide decomposition and. A classic. Catalyst Decomposition Example.
From www.youtube.com
Mechanism For N2O Catalytic Example Problem YouTube Catalyst Decomposition Example Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Enzyme inhibitors cause a decrease in the reaction rate of. An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2). Catalyst Decomposition Example.
From www.aakash.ac.in
reaction Definition, Classification, Uses and Importance Catalyst Decomposition Example See how to test catalysts using hydrogen peroxide decomposition and. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. One single reactant and two or more products; An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2].. Catalyst Decomposition Example.
From www.studocu.com
Simple CHEMICAL REACTION A reaction or Catalyst Decomposition Example Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2]. A classic example of this is an. A homogeneous catalyst is present in the same phase as the reactants.. Catalyst Decomposition Example.
From www.youtube.com
005 Types of Reactions YouTube Catalyst Decomposition Example Enzyme inhibitors cause a decrease in the reaction rate of. A classic example of this is an. A homogeneous catalyst is present in the same phase as the reactants. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Includes kit list and safety instructions. An example of a decomposition. Catalyst Decomposition Example.
From www.researchgate.net
Scheme II Reaction scheme for the catalytic of hydrogen Catalyst Decomposition Example Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2]. A classic example of. Catalyst Decomposition Example.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Decomposition Example H 2 co 3 (aq.) → co 2 (g) + h 2 o (l) Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. One single reactant and two or more products; An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon. Catalyst Decomposition Example.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Decomposition Example See how to test catalysts using hydrogen peroxide decomposition and. An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2]. Includes kit list and safety instructions. A homogeneous catalyst is present in the same phase as the reactants. One single reactant and two. Catalyst Decomposition Example.
From www.pinterest.com.au
What Is a Reaction? Definition and Examples Chemistry Catalyst Decomposition Example A classic example of this is an. Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. Includes kit list and safety instructions. It interacts with a reactant to form an intermediate substance, which then. A homogeneous catalyst is present in the same phase as the reactants. An example of a decomposition reaction is the breakdown. Catalyst Decomposition Example.
From www.researchgate.net
Catalytic Mechanism for Formaldehyde Download Catalyst Decomposition Example Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. It interacts with a reactant to form an intermediate substance, which then. One single reactant and two or more products; H 2 co 3 (aq.) → co 2 (g) + h 2 o (l) See how to test catalysts using hydrogen peroxide decomposition and. A classic. Catalyst Decomposition Example.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Decomposition Example See how to test catalysts using hydrogen peroxide decomposition and. H 2 co 3 (aq.) → co 2 (g) + h 2 o (l) Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. One single reactant and two or more products; An example of a decomposition reaction is the breakdown of carbonic acid (h 2. Catalyst Decomposition Example.
From www.numerade.com
SOLVED A number of reactions that take place on the surfaces of Catalyst Decomposition Example Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Catalytic decomposition is when a decomposition reaction occurs with the aide of a catalyst. It interacts with a reactant to form an intermediate substance, which then. Includes kit list and safety instructions. An example of a decomposition reaction is the. Catalyst Decomposition Example.
From www.pw.live
Reaction Definition, Formulas, Notes, Examples Catalyst Decomposition Example See how to test catalysts using hydrogen peroxide decomposition and. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. It interacts with a reactant to form an intermediate substance, which then. One single reactant and two or more products; A homogeneous catalyst is present in the same phase as. Catalyst Decomposition Example.
From www.researchgate.net
Comparison of the catalytic activity of catalyst with Catalyst Decomposition Example Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. H 2 co 3 (aq.) → co 2 (g) + h 2 o (l) An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2]. Catalytic decomposition is when. Catalyst Decomposition Example.
From scienceinfo.com
Reaction 3 Important Types, Uses, Examples Catalyst Decomposition Example Enzyme inhibitors cause a decrease in the reaction rate of. See how to test catalysts using hydrogen peroxide decomposition and. An example of a decomposition reaction is the breakdown of carbonic acid (h 2 co 3) to carbon dioxide (co 2) and water (h 2 o) [2]. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying. Catalyst Decomposition Example.