How To Perform Dilutions In Lab . Follow these five steps to make a dilution: This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. 50 μl per well * 2 for. Use the spectrophotometer to measure the absorbance of solutions. Calculate the minimum diluent volume per step: If you do not know them already, use the dilution factor equation to. A serial dilution is the repeated dilution of a solution to amplify the. To make a dilution series, use the following formulas: Several laboratory techniques and assays require to prepare serial dilutions. A dilution in chemistry is a process that reduces the concentration of a substance in a solution. This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the technique's. Generate a standard curve and use the standard curve to determine the concentration of.
from labpedia.net
If you do not know them already, use the dilution factor equation to. A serial dilution is the repeated dilution of a solution to amplify the. Generate a standard curve and use the standard curve to determine the concentration of. 50 μl per well * 2 for. Several laboratory techniques and assays require to prepare serial dilutions. Follow these five steps to make a dilution: This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. A dilution in chemistry is a process that reduces the concentration of a substance in a solution. Calculate the minimum diluent volume per step: This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the technique's.
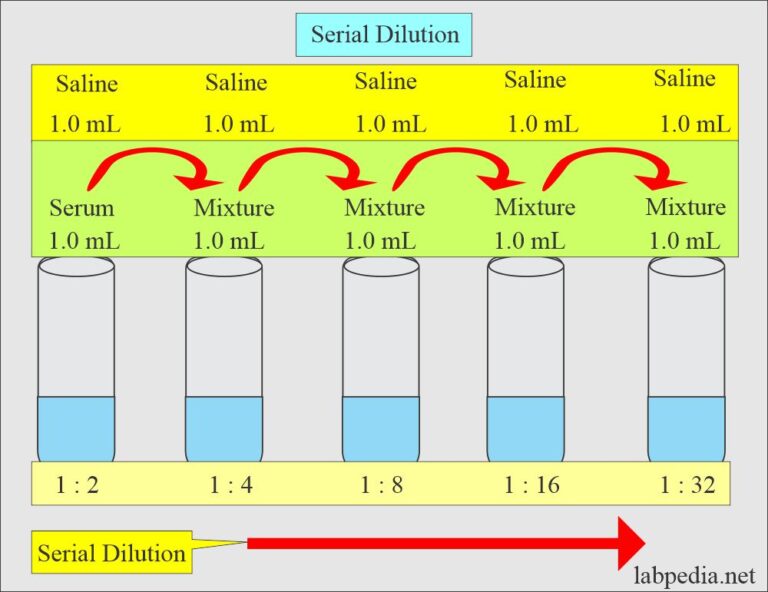
Solutions Part 1 Solutions Preparation used in Clinical Laboratory
How To Perform Dilutions In Lab Generate a standard curve and use the standard curve to determine the concentration of. If you do not know them already, use the dilution factor equation to. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the technique's. Use the spectrophotometer to measure the absorbance of solutions. Follow these five steps to make a dilution: To make a dilution series, use the following formulas: Calculate the minimum diluent volume per step: Generate a standard curve and use the standard curve to determine the concentration of. 50 μl per well * 2 for. A serial dilution is the repeated dilution of a solution to amplify the. A dilution in chemistry is a process that reduces the concentration of a substance in a solution. Several laboratory techniques and assays require to prepare serial dilutions.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube How To Perform Dilutions In Lab Use the spectrophotometer to measure the absorbance of solutions. This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the technique's. Generate a standard curve and use the standard curve to determine the concentration of. A dilution in chemistry is a process that reduces the. How To Perform Dilutions In Lab.
From www.slideshare.net
How to perform a two fold dilution in a clinical laboratory PDF How To Perform Dilutions In Lab To make a dilution series, use the following formulas: Generate a standard curve and use the standard curve to determine the concentration of. Calculate the minimum diluent volume per step: This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Several laboratory techniques and assays require to. How To Perform Dilutions In Lab.
From www.youtube.com
Dilutions and Lab Procedure (AP Chemistry) YouTube How To Perform Dilutions In Lab Calculate the minimum diluent volume per step: Generate a standard curve and use the standard curve to determine the concentration of. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Use the spectrophotometer to measure the absorbance of solutions. Several laboratory techniques and assays require to. How To Perform Dilutions In Lab.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory How To Perform Dilutions In Lab 50 μl per well * 2 for. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Follow these five steps to make a dilution: Generate a standard curve and use the standard curve to determine the concentration of. If you do not know them already, use. How To Perform Dilutions In Lab.
From www.slideserve.com
PPT AntigenAntibody Reaction Serial Dilution Technique PowerPoint How To Perform Dilutions In Lab Use the spectrophotometer to measure the absorbance of solutions. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the technique's. Generate. How To Perform Dilutions In Lab.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube How To Perform Dilutions In Lab 50 μl per well * 2 for. A dilution in chemistry is a process that reduces the concentration of a substance in a solution. This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the technique's. This guide will describe the process for preparing aqueous. How To Perform Dilutions In Lab.
From www.pinterest.com
How to Do Serial Dilutions 9 Steps (with Pictures) Biology labs How To Perform Dilutions In Lab Calculate the minimum diluent volume per step: A serial dilution is the repeated dilution of a solution to amplify the. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Follow these five steps to make a dilution: 50 μl per well * 2 for. A dilution. How To Perform Dilutions In Lab.
From www.youtube.com
How to Perform a Bacterial Dilution Calculation YouTube How To Perform Dilutions In Lab Generate a standard curve and use the standard curve to determine the concentration of. 50 μl per well * 2 for. Use the spectrophotometer to measure the absorbance of solutions. A serial dilution is the repeated dilution of a solution to amplify the. Calculate the minimum diluent volume per step: To make a dilution series, use the following formulas: Follow. How To Perform Dilutions In Lab.
From www.youtube.com
How to Do a Serial Dilution (9mL test tubes) MCCC Microbiology YouTube How To Perform Dilutions In Lab A dilution in chemistry is a process that reduces the concentration of a substance in a solution. Several laboratory techniques and assays require to prepare serial dilutions. Generate a standard curve and use the standard curve to determine the concentration of. Follow these five steps to make a dilution: This article describes what a serial dilution is, provides examples of. How To Perform Dilutions In Lab.
From www.researchgate.net
Procedures of serial dilution preparation Download Scientific Diagram How To Perform Dilutions In Lab To make a dilution series, use the following formulas: 50 μl per well * 2 for. Generate a standard curve and use the standard curve to determine the concentration of. If you do not know them already, use the dilution factor equation to. A dilution in chemistry is a process that reduces the concentration of a substance in a solution.. How To Perform Dilutions In Lab.
From www.youtube.com
How to create serial dilutions YouTube How To Perform Dilutions In Lab A dilution in chemistry is a process that reduces the concentration of a substance in a solution. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Calculate the minimum diluent volume per step: 50 μl per well * 2 for. Several laboratory techniques and assays require. How To Perform Dilutions In Lab.
From www.vrogue.co
Infographic Lab Basics How To Perform Serial Dilutions Medical Vrogue How To Perform Dilutions In Lab Several laboratory techniques and assays require to prepare serial dilutions. Follow these five steps to make a dilution: A serial dilution is the repeated dilution of a solution to amplify the. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. To make a dilution series, use. How To Perform Dilutions In Lab.
From www.youtube.com
How to Perform Serial Dilutions in Microbiology YouTube How To Perform Dilutions In Lab A serial dilution is the repeated dilution of a solution to amplify the. A dilution in chemistry is a process that reduces the concentration of a substance in a solution. Use the spectrophotometer to measure the absorbance of solutions. 50 μl per well * 2 for. To make a dilution series, use the following formulas: Follow these five steps to. How To Perform Dilutions In Lab.
From www.wikihow.com
How to Do Serial Dilutions 9 Steps (with Pictures) wikiHow How To Perform Dilutions In Lab Calculate the minimum diluent volume per step: To make a dilution series, use the following formulas: If you do not know them already, use the dilution factor equation to. Several laboratory techniques and assays require to prepare serial dilutions. Generate a standard curve and use the standard curve to determine the concentration of. A dilution in chemistry is a process. How To Perform Dilutions In Lab.
From ecampusontario.pressbooks.pub
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual How To Perform Dilutions In Lab If you do not know them already, use the dilution factor equation to. Follow these five steps to make a dilution: Use the spectrophotometer to measure the absorbance of solutions. Calculate the minimum diluent volume per step: This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well. How To Perform Dilutions In Lab.
From www.solutionspile.com
[Solved] View the dilution in your lab manual for How To Perform Dilutions In Lab This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. 50 μl per well * 2 for. Use the spectrophotometer to measure the absorbance of solutions. This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution,. How To Perform Dilutions In Lab.
From www.slideshare.net
How to perform a two fold dilution in a clinical laboratory How To Perform Dilutions In Lab A serial dilution is the repeated dilution of a solution to amplify the. This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the technique's. 50 μl per well * 2 for. If you do not know them already, use the dilution factor equation to.. How To Perform Dilutions In Lab.
From www.youtube.com
Serial dilutions of a bacterial culture (view at 1.25X) YouTube How To Perform Dilutions In Lab Use the spectrophotometer to measure the absorbance of solutions. Generate a standard curve and use the standard curve to determine the concentration of. This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the technique's. Several laboratory techniques and assays require to prepare serial dilutions.. How To Perform Dilutions In Lab.
From www.vrogue.co
Infographic Lab Basics How To Perform Serial Dilutions Medical Vrogue How To Perform Dilutions In Lab If you do not know them already, use the dilution factor equation to. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. A dilution in chemistry is a process that reduces the concentration of a substance in a solution. Calculate the minimum diluent volume per step:. How To Perform Dilutions In Lab.
From www.youtube.com
How to Perform Serial Dilution for Bacterial Growth Measurement Step How To Perform Dilutions In Lab Follow these five steps to make a dilution: Use the spectrophotometer to measure the absorbance of solutions. If you do not know them already, use the dilution factor equation to. To make a dilution series, use the following formulas: A serial dilution is the repeated dilution of a solution to amplify the. 50 μl per well * 2 for. Calculate. How To Perform Dilutions In Lab.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina How To Perform Dilutions In Lab Several laboratory techniques and assays require to prepare serial dilutions. To make a dilution series, use the following formulas: Use the spectrophotometer to measure the absorbance of solutions. This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the technique's. Generate a standard curve and. How To Perform Dilutions In Lab.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples How To Perform Dilutions In Lab Several laboratory techniques and assays require to prepare serial dilutions. Calculate the minimum diluent volume per step: 50 μl per well * 2 for. To make a dilution series, use the following formulas: Follow these five steps to make a dilution: This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a. How To Perform Dilutions In Lab.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory How To Perform Dilutions In Lab Generate a standard curve and use the standard curve to determine the concentration of. Follow these five steps to make a dilution: This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the technique's. 50 μl per well * 2 for. Several laboratory techniques and. How To Perform Dilutions In Lab.
From www.labmanager.com
How to Make and Dilute Aqueous Solutions Lab Manager How To Perform Dilutions In Lab This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the technique's. Calculate the minimum diluent volume per step: Generate a standard curve and use the standard curve to determine the concentration of. If you do not know them already, use the dilution factor equation. How To Perform Dilutions In Lab.
From www.youtube.com
Serial Dilution Technique For Microbiological & Chemical Analysis How To Perform Dilutions In Lab Several laboratory techniques and assays require to prepare serial dilutions. 50 μl per well * 2 for. Calculate the minimum diluent volume per step: Use the spectrophotometer to measure the absorbance of solutions. A serial dilution is the repeated dilution of a solution to amplify the. This article describes what a serial dilution is, provides examples of common applications, and. How To Perform Dilutions In Lab.
From microbeonline.com
Serial Dilution Method for Estimating Viable Count of Bacteria How To Perform Dilutions In Lab Follow these five steps to make a dilution: A serial dilution is the repeated dilution of a solution to amplify the. 50 μl per well * 2 for. This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the technique's. If you do not know. How To Perform Dilutions In Lab.
From www.youtube.com
HOW TO MAKE DILUTION IN LAB DILUTION KYA HOTA HAI DILUTION IN How To Perform Dilutions In Lab A serial dilution is the repeated dilution of a solution to amplify the. Several laboratory techniques and assays require to prepare serial dilutions. Generate a standard curve and use the standard curve to determine the concentration of. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume.. How To Perform Dilutions In Lab.
From microbeonline.com
Serial Dilution Method for Estimating Viable Count of Bacteria How To Perform Dilutions In Lab 50 μl per well * 2 for. If you do not know them already, use the dilution factor equation to. Calculate the minimum diluent volume per step: This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the technique's. A dilution in chemistry is a. How To Perform Dilutions In Lab.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory How To Perform Dilutions In Lab If you do not know them already, use the dilution factor equation to. To make a dilution series, use the following formulas: Generate a standard curve and use the standard curve to determine the concentration of. 50 μl per well * 2 for. This article describes what a serial dilution is, provides examples of common applications, and explains how to. How To Perform Dilutions In Lab.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in How To Perform Dilutions In Lab Several laboratory techniques and assays require to prepare serial dilutions. To make a dilution series, use the following formulas: This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the technique's. Calculate the minimum diluent volume per step: Generate a standard curve and use the. How To Perform Dilutions In Lab.
From www.vrogue.co
Infographic Lab Basics How To Perform Serial Dilutions Medical Vrogue How To Perform Dilutions In Lab A dilution in chemistry is a process that reduces the concentration of a substance in a solution. To make a dilution series, use the following formulas: 50 μl per well * 2 for. A serial dilution is the repeated dilution of a solution to amplify the. Generate a standard curve and use the standard curve to determine the concentration of.. How To Perform Dilutions In Lab.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts How To Perform Dilutions In Lab This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. To make a dilution series, use the following formulas: Calculate the minimum diluent volume per step: Generate a standard curve and use the standard curve to determine the concentration of. This article describes what a serial dilution. How To Perform Dilutions In Lab.
From www.slideshare.net
How to perform a two fold dilution in a clinical laboratory How To Perform Dilutions In Lab If you do not know them already, use the dilution factor equation to. A dilution in chemistry is a process that reduces the concentration of a substance in a solution. Use the spectrophotometer to measure the absorbance of solutions. Several laboratory techniques and assays require to prepare serial dilutions. This guide will describe the process for preparing aqueous solutions, including. How To Perform Dilutions In Lab.
From study.com
Serial Dilution in Microbiology Calculation, Method & Technique How To Perform Dilutions In Lab Follow these five steps to make a dilution: This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. This article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the technique's. 50 μl. How To Perform Dilutions In Lab.
From www.vrogue.co
What Is Serial Dilution Method And How To Calculate S vrogue.co How To Perform Dilutions In Lab Several laboratory techniques and assays require to prepare serial dilutions. Follow these five steps to make a dilution: This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. 50 μl per well * 2 for. Use the spectrophotometer to measure the absorbance of solutions. A dilution in. How To Perform Dilutions In Lab.