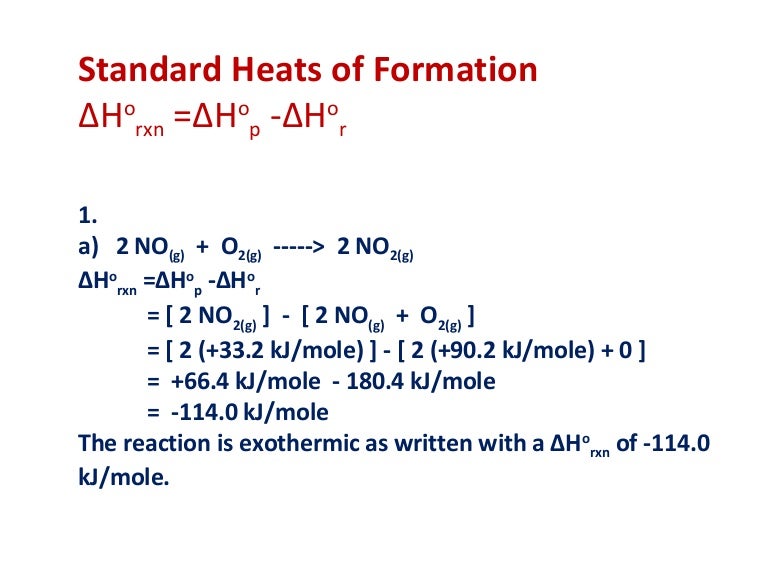
Standard Enthalpy Of Formation To . A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The symbol for the standard enthalpy of formation is: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. All chemical reactions involve a change in enthalpy (defined as the heat produced or. The formation of any chemical can be as a reaction from the corresponding elements: The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. \ [ \text {elements} \rightarrow \text {compound} \nonumber\]
from www.slideshare.net
A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The symbol for the standard enthalpy of formation is: All chemical reactions involve a change in enthalpy (defined as the heat produced or. \ [ \text {elements} \rightarrow \text {compound} \nonumber\] The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The formation of any chemical can be as a reaction from the corresponding elements:
Standard enthalpy of formation
Standard Enthalpy Of Formation To The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. All chemical reactions involve a change in enthalpy (defined as the heat produced or. \ [ \text {elements} \rightarrow \text {compound} \nonumber\] The symbol for the standard enthalpy of formation is: The formation of any chemical can be as a reaction from the corresponding elements: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure.
From
Standard Enthalpy Of Formation To A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. \ [ \text {elements} \rightarrow \text {compound} \nonumber\] Enthalpy of. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The symbol for. Standard Enthalpy Of Formation To.
From www.vrogue.co
Standard Enthalpy Of Formation And Standard Free Ener vrogue.co Standard Enthalpy Of Formation To \ [ \text {elements} \rightarrow \text {compound} \nonumber\] Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The formation of any chemical can be as a reaction from the corresponding elements: A standard enthalpy of formation. Standard Enthalpy Of Formation To.
From www.studocu.com
Standard Enthalpies of Formation & Standard Entropies kJ J ( mol Standard Enthalpy Of Formation To 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. All chemical reactions involve a change in enthalpy (defined as the heat produced. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To The formation of any chemical can be as a reaction from the corresponding elements: A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. \ [ \text {elements} \rightarrow \text {compound} \nonumber\] The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation,. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. \ [ \text {elements} \rightarrow \text {compound} \nonumber\] Enthalpy of formation (\ (δh_f\)) is the enthalpy. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To The formation of any chemical can be as a reaction from the corresponding elements: Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction. Standard Enthalpy Of Formation To.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID6319219 Standard Enthalpy Of Formation To A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. All chemical reactions involve a change in enthalpy (defined as the heat produced or. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, δh o f, is the enthalpy. Standard Enthalpy Of Formation To.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5072129 Standard Enthalpy Of Formation To The formation of any chemical can be as a reaction from the corresponding elements: \ [ \text {elements} \rightarrow \text {compound} \nonumber\] The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The formation of any chemical can be as a reaction from the corresponding elements: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change. Standard Enthalpy Of Formation To.
From radenbayu33as.blogspot.com
Enthalpy Of Formation Of C3H8 Enthalpy Changes during Formation of Standard Enthalpy Of Formation To 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The formation of any chemical can be as a reaction from the corresponding. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or. Standard Enthalpy Of Formation To.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation To A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The formation of any chemical can be as a reaction from the corresponding elements: The standard. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The symbol for the standard enthalpy of formation is: \ [ \text {elements} \rightarrow \text. Standard Enthalpy Of Formation To.
From rayb78.github.io
Heat Of Formation Chart Standard Enthalpy Of Formation To The symbol for the standard enthalpy of formation is: A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. All chemical reactions involve a change in enthalpy (defined as the heat produced or. The formation of any chemical can be as a reaction from the corresponding elements: The standard. Standard Enthalpy Of Formation To.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation To A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The formation of any chemical can be as a reaction from. Standard Enthalpy Of Formation To.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation To All chemical reactions involve a change in enthalpy (defined as the heat produced or. \ [ \text {elements} \rightarrow \text {compound} \nonumber\] The symbol for the standard enthalpy of formation is: A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation, δh o f,. Standard Enthalpy Of Formation To.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation To Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. All chemical reactions. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. The symbol for the standard enthalpy of formation is: Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To The symbol for the standard enthalpy of formation is: \ [ \text {elements} \rightarrow \text {compound} \nonumber\] A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. All chemical reactions involve a change in enthalpy (defined as. Standard Enthalpy Of Formation To.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation To A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. All chemical reactions involve a change in enthalpy (defined as the heat produced or. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the. Standard Enthalpy Of Formation To.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free Standard Enthalpy Of Formation To A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of. Standard Enthalpy Of Formation To.
From mavink.com
Standard Enthalpy Chart Standard Enthalpy Of Formation To The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. The formation of any chemical can be as a reaction from the corresponding elements: All chemical reactions involve a change in enthalpy (defined as the heat produced or. 193 rows in chemistry and thermodynamics, the standard. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To All chemical reactions involve a change in enthalpy (defined as the heat produced or. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are. Standard Enthalpy Of Formation To.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation To The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. All chemical reactions involve a change in enthalpy (defined as the heat produced or. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements,. Standard Enthalpy Of Formation To.
From pt.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation To \ [ \text {elements} \rightarrow \text {compound} \nonumber\] The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. All chemical reactions involve a change in enthalpy (defined as the heat produced or. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. The formation of any chemical can be as a reaction from the corresponding elements: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly. Standard Enthalpy Of Formation To.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation To A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The symbol for the standard enthalpy of formation is: \. Standard Enthalpy Of Formation To.
From
Standard Enthalpy Of Formation To The formation of any chemical can be as a reaction from the corresponding elements: \ [ \text {elements} \rightarrow \text {compound} \nonumber\] 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The symbol for the standard enthalpy of formation is: A standard enthalpy of formation \(δh^\circ_\ce{f}\). Standard Enthalpy Of Formation To.