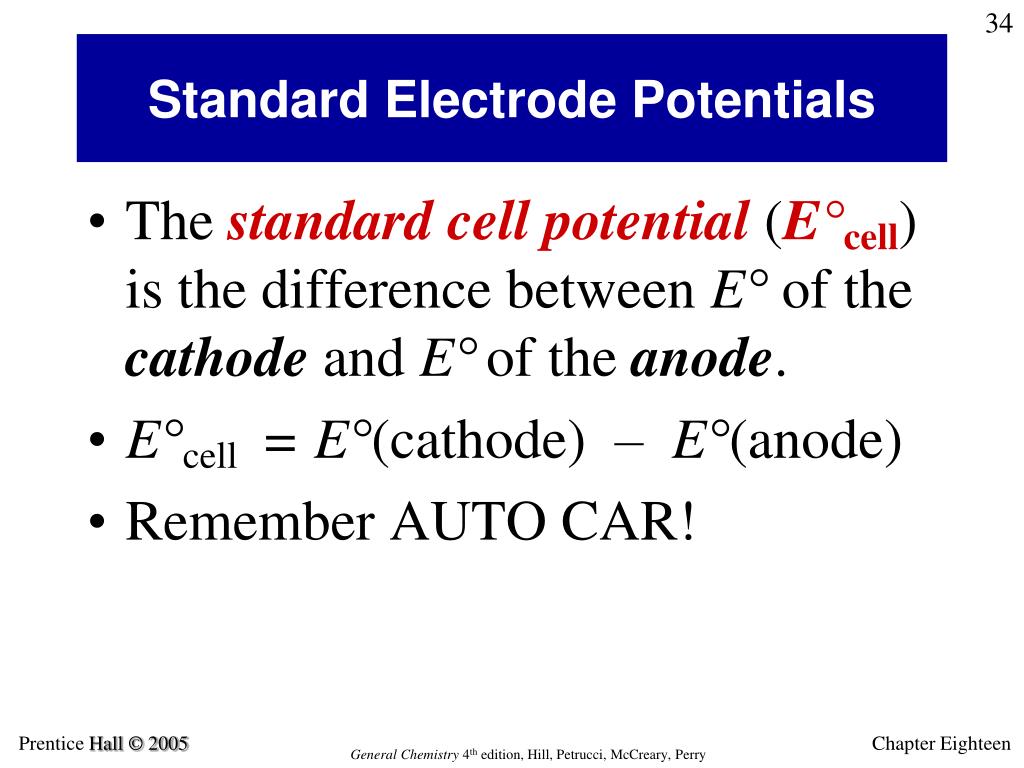
Standard Electrode Potential How . The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Describe and relate the definitions of electrode and cell potentials. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. Interpret electrode potentials in terms of relative oxidant and reductant.
from www.slideserve.com
Interpret electrode potentials in terms of relative oxidant and reductant. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Describe and relate the definitions of electrode and cell potentials. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard.
PPT Electrochemistry PowerPoint Presentation, free download ID1937020
Standard Electrode Potential How Interpret electrode potentials in terms of relative oxidant and reductant. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion.
From chem.libretexts.org
9.4 Standard Electrode Potentials Chemistry LibreTexts Standard Electrode Potential How Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions. Standard Electrode Potential How.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Electrode Potential How Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere,. Standard Electrode Potential How.
From www.youtube.com
19.1 Calculate cell potentials using standard electrode potentials [HL Standard Electrode Potential How Interpret electrode potentials in terms of relative oxidant and reductant. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere,. Standard Electrode Potential How.
From scienceinfo.com
Measuring the Standard Electrode Potential (Eꝋ) Standard Electrode Potential How The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Describe and relate the definitions of electrode. Standard Electrode Potential How.
From www.youtube.com
Electrode Potential Standard Electrode potential Reduction potential Standard Electrode Potential How The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. Interpret electrode potentials in terms of relative oxidant and reductant. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal. Standard Electrode Potential How.
From www.flexiprep.com
Electrochemistry Electrode Potential and Standard Electrode Potential Standard Electrode Potential How Interpret electrode potentials in terms of relative oxidant and reductant. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal. Standard Electrode Potential How.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Standard Electrode Potential How Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions. Standard Electrode Potential How.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Electrode Potential How Interpret electrode potentials in terms of relative oxidant and reductant. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Describe and relate the definitions of electrode and cell potentials. The standard electrode potentials are customarily determined at. Standard Electrode Potential How.
From mungfali.com
Standard Electrode Potential Table Standard Electrode Potential How The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions. Standard Electrode Potential How.
From question.pandai.org
Standard Electrode Potential Standard Electrode Potential How The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Interpret electrode potentials in terms of relative oxidant and reductant. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere,. Standard Electrode Potential How.
From www.slideserve.com
PPT Electrochemistry II PowerPoint Presentation, free download ID Standard Electrode Potential How The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. Interpret electrode potentials in terms of relative oxidant and reductant. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal. Standard Electrode Potential How.
From www.youtube.com
Using Standard Electrode Potentials YouTube Standard Electrode Potential How Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Interpret electrode potentials in terms of relative oxidant and reductant. The standard electrode potentials are customarily determined at. Standard Electrode Potential How.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential How The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Describe and relate the definitions of electrode and cell potentials. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere,. Standard Electrode Potential How.
From www.pveducation.org
Standard Potential PVEducation Standard Electrode Potential How The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. Describe and relate the definitions of electrode. Standard Electrode Potential How.
From byjus.com
6. The standard electrode potential E^° I2/I , E^° Br /Br2 and E^° Fe Standard Electrode Potential How Interpret electrode potentials in terms of relative oxidant and reductant. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Describe and relate the definitions of electrode and cell potentials. The standard electrode potentials are customarily determined at. Standard Electrode Potential How.
From www.substech.com
standard_electrode_potential.png [SubsTech] Standard Electrode Potential How The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. The standard electrode potentials are customarily determined at. Standard Electrode Potential How.
From users.highland.edu
Standard Potentials Standard Electrode Potential How The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. Describe and relate the definitions of electrode. Standard Electrode Potential How.
From meteonmendez.blogspot.com
Standard Electrode Potential Table MeteonMendez Standard Electrode Potential How The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. The standard electrode potentials are customarily determined at. Standard Electrode Potential How.
From www.slideserve.com
PPT Electrochemical Cells Voltage (Electric potential) PowerPoint Standard Electrode Potential How Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere,. Standard Electrode Potential How.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Standard Electrode Potential How Interpret electrode potentials in terms of relative oxidant and reductant. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions. Standard Electrode Potential How.
From www.chemistryland.com
Lab 8 Single Replacement Reactions Standard Electrode Potential How Interpret electrode potentials in terms of relative oxidant and reductant. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere,. Standard Electrode Potential How.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Standard Electrode Potential How Interpret electrode potentials in terms of relative oxidant and reductant. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal. Standard Electrode Potential How.
From www.youtube.com
19.1 Standard electrode potential (HL) YouTube Standard Electrode Potential How Interpret electrode potentials in terms of relative oxidant and reductant. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions. Standard Electrode Potential How.
From exowfmfux.blob.core.windows.net
Standard Electrode Examples at Lucille Fitzsimmons blog Standard Electrode Potential How Describe and relate the definitions of electrode and cell potentials. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal. Standard Electrode Potential How.
From mungfali.com
Electrode Potential Series Standard Electrode Potential How The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Interpret electrode potentials in terms of relative oxidant and reductant. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere,. Standard Electrode Potential How.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Electrode Potential How The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The standard electrode potentials are customarily determined at. Standard Electrode Potential How.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Standard Electrode Potential How Describe and relate the definitions of electrode and cell potentials. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal. Standard Electrode Potential How.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential How Describe and relate the definitions of electrode and cell potentials. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. Interpret electrode potentials in terms of relative oxidant and reductant. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions. Standard Electrode Potential How.
From www.youtube.com
APSC132 lecture 5 03 Standard Electrode Potential YouTube Standard Electrode Potential How Describe and relate the definitions of electrode and cell potentials. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal. Standard Electrode Potential How.
From question.pandai.org
Standard Electrode Potential Standard Electrode Potential How Interpret electrode potentials in terms of relative oxidant and reductant. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal. Standard Electrode Potential How.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 Standard Electrode Potential How The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. Interpret electrode potentials in terms of relative. Standard Electrode Potential How.
From app.pandai.org
Standard Electrode Potential Standard Electrode Potential How The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Interpret electrode potentials in terms of relative. Standard Electrode Potential How.
From cemubadx.blob.core.windows.net
Standard Cell Potential And Standard Electrode Potential at Nina Standard Electrode Potential How The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions. Standard Electrode Potential How.
From 2012books.lardbucket.org
Standard Potentials Standard Electrode Potential How The standard electrode potentials are customarily determined at solute concentrations of 1 molar, gas pressures of 1 atmosphere, and a standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Interpret electrode potentials in terms of relative. Standard Electrode Potential How.
From cbsencertsolutiononline.blogspot.com
CBSE NCERT SOLUTIONS The standard electrode potentials at 298 K Standard Electrode Potential How The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. The standard electrode potentials are customarily determined at. Standard Electrode Potential How.