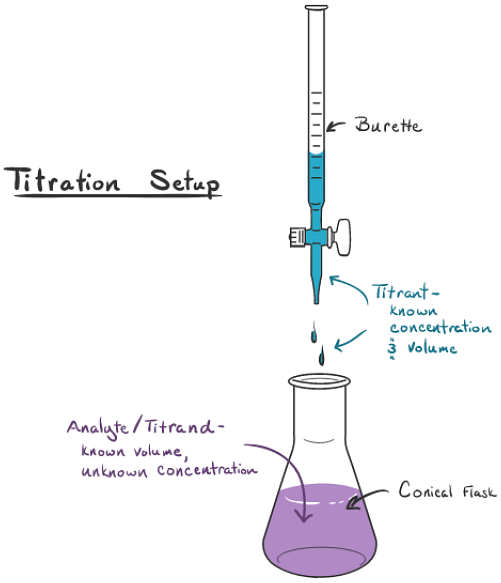
Indicator For Direct Titration . Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. This page assumes that you know about ph curves for all the.
from collegedunia.com
We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. This page assumes that you know about ph curves for all the. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order.
Titration Formula Types, Process & Acid Base Indicators
Indicator For Direct Titration In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. This page assumes that you know about ph curves for all the. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order.
From sciencing.com
What Is "Direct Titration"? Sciencing Indicator For Direct Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence.. Indicator For Direct Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Indicator For Direct Titration Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. This page assumes that you know about ph curves for all the. In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. We can use this change in color to. Indicator For Direct Titration.
From www.youtube.com
Choosing an INDICATOR for a titration YouTube Indicator For Direct Titration Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100. Indicator For Direct Titration.
From www.science-revision.co.uk
Titrations Indicator For Direct Titration This page assumes that you know about ph curves for all the. In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence.. Indicator For Direct Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicator For Direct Titration We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. This page assumes that you know about ph curves for all the. The graph shows the results obtained. Indicator For Direct Titration.
From exoraquci.blob.core.windows.net
List Of Indicators Used In Titration at Jennifer Bowman blog Indicator For Direct Titration We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red,. Indicator For Direct Titration.
From exordpaul.blob.core.windows.net
Titration For Indicators at Robert Creech blog Indicator For Direct Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. We can use this change in color to. Indicator For Direct Titration.
From exordpaul.blob.core.windows.net
Titration For Indicators at Robert Creech blog Indicator For Direct Titration In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. This page assumes that you know about ph curves for all the.. Indicator For Direct Titration.
From mungfali.com
Acid Base Titration Indicator Indicator For Direct Titration This page assumes that you know about ph curves for all the. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. We can use this change in color to indicate the end point of. Indicator For Direct Titration.
From www.feedback-shop.co.uk
Selecting an indicator for titration Titrations Acids and bases Indicator For Direct Titration Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. We can use this change in color to. Indicator For Direct Titration.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Indicator For Direct Titration We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. This page assumes that you know about ph curves for all the. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong. Indicator For Direct Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Indicator For Direct Titration Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. We can use this change in color to indicate the end point of a titration provided that it occurs at. Indicator For Direct Titration.
From mmerevise.co.uk
pH Curves Questions and Revision MME Indicator For Direct Titration We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. This page assumes that you know about ph curves for all the.. Indicator For Direct Titration.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation Indicator For Direct Titration In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. This page assumes that you know about ph curves for all the.. Indicator For Direct Titration.
From dxonbtcto.blob.core.windows.net
Chemical Indicator AcidBase Titration at Hilda Johnston blog Indicator For Direct Titration This page assumes that you know about ph curves for all the. In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl). Indicator For Direct Titration.
From www.feedback-shop.co.uk
Selecting an indicator for titration Digital Titrations Acids and Indicator For Direct Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. This page assumes that you know about ph curves for all the. We can use this change in color to indicate the end point of. Indicator For Direct Titration.
From dokumen.tips
(PDF) Modified Methyl Yellow Indicator for Direct Titration of Sodium Indicator For Direct Titration In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red,. Indicator For Direct Titration.
From exordpaul.blob.core.windows.net
Titration For Indicators at Robert Creech blog Indicator For Direct Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence.. Indicator For Direct Titration.
From wisc.pb.unizin.org
D30.1 AcidBase Indicators Chemistry 109 Fall 2021 Indicator For Direct Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. This page assumes that you know about ph curves for all the. We can use this change in color to indicate the end point of. Indicator For Direct Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Indicator For Direct Titration Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. This page assumes that you know about ph. Indicator For Direct Titration.
From cezsemit.blob.core.windows.net
Why Are Different Indicators Used In Titration at Ladonna Anderson blog Indicator For Direct Titration In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Some commonly. Indicator For Direct Titration.
From joiyfxbtq.blob.core.windows.net
Titration Method Image at Jodie Massey blog Indicator For Direct Titration This page assumes that you know about ph curves for all the. We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. In most manufacturing or processing industries,. Indicator For Direct Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 Indicator For Direct Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. This page assumes that you know about ph curves for all the. In most manufacturing or processing industries, it is essential to know the exact. Indicator For Direct Titration.
From freesvg.org
Redox Titration Using Indicator Free SVG Indicator For Direct Titration We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh.. Indicator For Direct Titration.
From www.researchgate.net
Indicators for direct complexometric titration of bismuth Download Table Indicator For Direct Titration This page assumes that you know about ph curves for all the. In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl). Indicator For Direct Titration.
From mungfali.com
Acid Base Titration Indicator Indicator For Direct Titration This page assumes that you know about ph curves for all the. We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong. Indicator For Direct Titration.
From exordpaul.blob.core.windows.net
Titration For Indicators at Robert Creech blog Indicator For Direct Titration We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. This page assumes that you know about ph curves for all the. In most manufacturing or processing industries,. Indicator For Direct Titration.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Indicator For Direct Titration This page assumes that you know about ph curves for all the. We can use this change in color to indicate the end point of a titration provided that it occurs at or near the titration’s equivalence. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. In most manufacturing or processing industries,. Indicator For Direct Titration.
From jeparatd.blogspot.com
Why Does The Indicator Change Color In Titration Jeparat Indicator For Direct Titration Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. We can use this change in color to indicate the end point of a titration provided that it occurs at. Indicator For Direct Titration.
From www.scribd.com
Indicators Titration Chemistry Indicator For Direct Titration This page assumes that you know about ph curves for all the. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol. Indicator For Direct Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicator For Direct Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. Some commonly. Indicator For Direct Titration.
From gwb.ee
Titration indicators G.W.Berg OÜ LABORI JA ANALÜÜSISEADMED Indicator For Direct Titration In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. This page assumes that you know about ph curves for all the. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl). Indicator For Direct Titration.
From www.youtube.com
IGCSE Chemistry Titration and indicators method question YouTube Indicator For Direct Titration In most manufacturing or processing industries, it is essential to know the exact concentration of a product, species or chemical function in order. This page assumes that you know about ph curves for all the. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl). Indicator For Direct Titration.
From pediaa.com
Difference Between Back Titration and Direct Titration Definition Indicator For Direct Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. We can use this change in color to. Indicator For Direct Titration.
From collegedunia.com
Titration Formula Types, Process & Acid Base Indicators Indicator For Direct Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. This page assumes that you know about ph curves for all the. We can use this change in color to indicate the end point of. Indicator For Direct Titration.