Magnesium Ion That Has 10 Electrons Charge . When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. For example, when a neutral sodium atom loses one electron, it becomes the sodium ion, na + as shown in the following shorthand notation: The symbol for the ion is mg 2+, and it is called a magnesium ion. You can use this table to predict whether an atom can bond with another atom. This electric charge generated on the ion is known. The charge on a magnesium ion with ten electrons would be +2, since magnesium typically has 12 electrons in its neutral state. 93 rows this table shows the most common charges for atoms of the chemical elements. The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in. A magnesium ion therefore has 10 electrons.
from material-properties.org
A magnesium ion therefore has 10 electrons. This electric charge generated on the ion is known. The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in. For example, when a neutral sodium atom loses one electron, it becomes the sodium ion, na + as shown in the following shorthand notation: The symbol for the ion is mg 2+, and it is called a magnesium ion. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The charge on a magnesium ion with ten electrons would be +2, since magnesium typically has 12 electrons in its neutral state. You can use this table to predict whether an atom can bond with another atom. 93 rows this table shows the most common charges for atoms of the chemical elements.
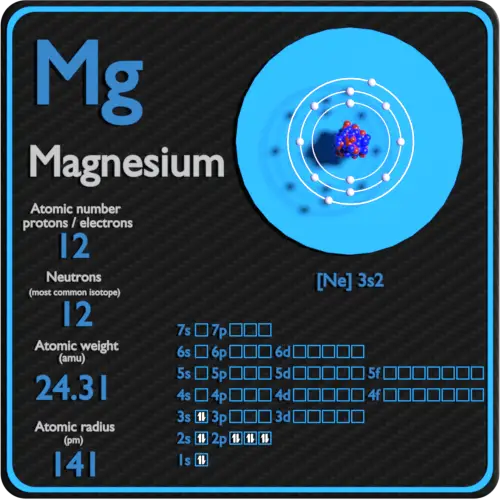
Magnesium Periodic Table and Atomic Properties
Magnesium Ion That Has 10 Electrons Charge The charge on a magnesium ion with ten electrons would be +2, since magnesium typically has 12 electrons in its neutral state. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). You can use this table to predict whether an atom can bond with another atom. The symbol for the ion is mg 2+, and it is called a magnesium ion. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. This electric charge generated on the ion is known. 93 rows this table shows the most common charges for atoms of the chemical elements. The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in. For example, when a neutral sodium atom loses one electron, it becomes the sodium ion, na + as shown in the following shorthand notation: The charge on a magnesium ion with ten electrons would be +2, since magnesium typically has 12 electrons in its neutral state. A magnesium ion therefore has 10 electrons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Ion That Has 10 Electrons Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in. For example, when a neutral sodium atom loses one electron, it becomes the sodium ion, na +. Magnesium Ion That Has 10 Electrons Charge.
From exoilgobw.blob.core.windows.net
Magnesium Ion Explanation at Helen Weeks blog Magnesium Ion That Has 10 Electrons Charge 93 rows this table shows the most common charges for atoms of the chemical elements. The charge on a magnesium ion with ten electrons would be +2, since magnesium typically has 12 electrons in its neutral state. The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in.. Magnesium Ion That Has 10 Electrons Charge.
From www.slideshare.net
The periodic table Magnesium Ion That Has 10 Electrons Charge Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. You can use this table to predict whether an atom can bond with another atom. The charge on a magnesium ion with ten electrons would be +2, since magnesium typically has 12 electrons in its neutral state. For example, when a. Magnesium Ion That Has 10 Electrons Charge.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Ion That Has 10 Electrons Charge This electric charge generated on the ion is known. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. You can use this table to predict whether an atom can bond. Magnesium Ion That Has 10 Electrons Charge.
From brainly.in
EXPLAIN WHY MAGNESIUM FORMS MG ION Brainly.in Magnesium Ion That Has 10 Electrons Charge 93 rows this table shows the most common charges for atoms of the chemical elements. The symbol for the ion is mg 2+, and it is called a magnesium ion. The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in. A magnesium ion therefore has 10 electrons.. Magnesium Ion That Has 10 Electrons Charge.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion That Has 10 Electrons Charge For example, when a neutral sodium atom loses one electron, it becomes the sodium ion, na + as shown in the following shorthand notation: This electric charge generated on the ion is known. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. You can use this table to predict whether. Magnesium Ion That Has 10 Electrons Charge.
From www.shutterstock.com
Magnesium Atom. Diagram Representation Of The Element Magnesium Magnesium Ion That Has 10 Electrons Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in. 93 rows this table shows the most common charges for atoms of the chemical elements. Thus, a. Magnesium Ion That Has 10 Electrons Charge.
From stock.adobe.com
Bohr model diagram of magnesium in atomic physics Stock Vector Adobe Magnesium Ion That Has 10 Electrons Charge The charge on a magnesium ion with ten electrons would be +2, since magnesium typically has 12 electrons in its neutral state. A magnesium ion therefore has 10 electrons. The symbol for the ion is mg 2+, and it is called a magnesium ion. This electric charge generated on the ion is known. 93 rows this table shows the most. Magnesium Ion That Has 10 Electrons Charge.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Magnesium Ion That Has 10 Electrons Charge The symbol for the ion is mg 2+, and it is called a magnesium ion. This electric charge generated on the ion is known. You can use this table to predict whether an atom can bond with another atom. A magnesium ion therefore has 10 electrons. The charge on a magnesium ion with ten electrons would be +2, since magnesium. Magnesium Ion That Has 10 Electrons Charge.
From www.thesciencehive.co.uk
Atomic Structure and Electron Configuration (AQA) — the science hive Magnesium Ion That Has 10 Electrons Charge 93 rows this table shows the most common charges for atoms of the chemical elements. The symbol for the ion is mg 2+, and it is called a magnesium ion. The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in. The charge on a magnesium ion with. Magnesium Ion That Has 10 Electrons Charge.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Ion That Has 10 Electrons Charge For example, when a neutral sodium atom loses one electron, it becomes the sodium ion, na + as shown in the following shorthand notation: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). You can use this table to predict whether an atom can bond with another atom. Thus,. Magnesium Ion That Has 10 Electrons Charge.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium Ion That Has 10 Electrons Charge The charge on a magnesium ion with ten electrons would be +2, since magnesium typically has 12 electrons in its neutral state. A magnesium ion therefore has 10 electrons. The symbol for the ion is mg 2+, and it is called a magnesium ion. You can use this table to predict whether an atom can bond with another atom. The. Magnesium Ion That Has 10 Electrons Charge.
From quizlet.com
Magnesium Oxide Diagram Quizlet Magnesium Ion That Has 10 Electrons Charge You can use this table to predict whether an atom can bond with another atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows this table shows the most common charges for atoms of the chemical elements. The symbol for the ion is mg 2+, and it. Magnesium Ion That Has 10 Electrons Charge.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion That Has 10 Electrons Charge A magnesium ion therefore has 10 electrons. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in. The symbol for the ion is mg 2+, and it is. Magnesium Ion That Has 10 Electrons Charge.
From mungfali.com
Magnesium Orbital Diagram Magnesium Ion That Has 10 Electrons Charge The charge on a magnesium ion with ten electrons would be +2, since magnesium typically has 12 electrons in its neutral state. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows this table shows the most common charges for atoms of the chemical elements. A magnesium ion. Magnesium Ion That Has 10 Electrons Charge.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion That Has 10 Electrons Charge This electric charge generated on the ion is known. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is mg 2+, and it is called a magnesium ion. You can use this table to predict whether an atom can bond with another atom. A magnesium. Magnesium Ion That Has 10 Electrons Charge.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Ion That Has 10 Electrons Charge 93 rows this table shows the most common charges for atoms of the chemical elements. The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in. This electric charge generated on the ion is known. A magnesium ion therefore has 10 electrons. The symbol for the ion is. Magnesium Ion That Has 10 Electrons Charge.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Ion That Has 10 Electrons Charge The charge on a magnesium ion with ten electrons would be +2, since magnesium typically has 12 electrons in its neutral state. This electric charge generated on the ion is known. For example, when a neutral sodium atom loses one electron, it becomes the sodium ion, na + as shown in the following shorthand notation: 93 rows this table shows. Magnesium Ion That Has 10 Electrons Charge.
From celbncbc.blob.core.windows.net
Magnesium Ion Number Of Electrons at Joan Harbert blog Magnesium Ion That Has 10 Electrons Charge For example, when a neutral sodium atom loses one electron, it becomes the sodium ion, na + as shown in the following shorthand notation: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). You can use this table to predict whether an atom can bond with another atom. This. Magnesium Ion That Has 10 Electrons Charge.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Ion That Has 10 Electrons Charge Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is mg 2+, and it is called a magnesium ion. A magnesium ion therefore has 10 electrons. 93 rows this table shows the most common charges for atoms of the chemical elements. This electric charge generated. Magnesium Ion That Has 10 Electrons Charge.
From guidedehartsculptures.z21.web.core.windows.net
Magnesium Electric Dot Diagram Magnesium Ion That Has 10 Electrons Charge The charge on a magnesium ion with ten electrons would be +2, since magnesium typically has 12 electrons in its neutral state. For example, when a neutral sodium atom loses one electron, it becomes the sodium ion, na + as shown in the following shorthand notation: This electric charge generated on the ion is known. The first shows common element. Magnesium Ion That Has 10 Electrons Charge.
From joiuszboj.blob.core.windows.net
Magnesium Electric Charge at Gary Kinney blog Magnesium Ion That Has 10 Electrons Charge The charge on a magnesium ion with ten electrons would be +2, since magnesium typically has 12 electrons in its neutral state. The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in. A magnesium ion therefore has 10 electrons. You can use this table to predict whether. Magnesium Ion That Has 10 Electrons Charge.
From symbiosis.co.nz
Magnesium Symbiosis Agriculture Magnesium Ion That Has 10 Electrons Charge Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). You can use this table to predict whether an atom can bond with another atom. This electric charge generated on the. Magnesium Ion That Has 10 Electrons Charge.
From valenceelectrons.com
How to Find the Valence Electrons for Magnesium (Mg)? Magnesium Ion That Has 10 Electrons Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge. Magnesium Ion That Has 10 Electrons Charge.
From nursehub.com
Electron Shells NurseHub Magnesium Ion That Has 10 Electrons Charge Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is mg 2+, and it is called a magnesium ion. The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in. This electric charge. Magnesium Ion That Has 10 Electrons Charge.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Ion That Has 10 Electrons Charge The symbol for the ion is mg 2+, and it is called a magnesium ion. For example, when a neutral sodium atom loses one electron, it becomes the sodium ion, na + as shown in the following shorthand notation: The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common. Magnesium Ion That Has 10 Electrons Charge.
From valenceelectrons.com
How Many Protons,Neutrons and Electrons Does Magnesium Have? Magnesium Ion That Has 10 Electrons Charge The charge on a magnesium ion with ten electrons would be +2, since magnesium typically has 12 electrons in its neutral state. A magnesium ion therefore has 10 electrons. The symbol for the ion is mg 2+, and it is called a magnesium ion. This electric charge generated on the ion is known. Thus, a magnesium atom will form a. Magnesium Ion That Has 10 Electrons Charge.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Ion That Has 10 Electrons Charge The symbol for the ion is mg 2+, and it is called a magnesium ion. You can use this table to predict whether an atom can bond with another atom. A magnesium ion therefore has 10 electrons. This electric charge generated on the ion is known. The first shows common element charges, while the second shows all the element charges. Magnesium Ion That Has 10 Electrons Charge.
From brainly.com
⚗️What is the electron configuration for the Magnesium ion? Magnesium Ion That Has 10 Electrons Charge The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in. The symbol for the ion is mg 2+, and it is called a magnesium ion. You can use this table to predict whether an atom can bond with another atom. This electric charge generated on the ion. Magnesium Ion That Has 10 Electrons Charge.
From slidetodoc.com
Metal ions Nonmetal ions Positive ion Gain electrons Magnesium Ion That Has 10 Electrons Charge Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. This electric charge generated on the ion is known. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). You can use this table to predict whether an atom can bond. Magnesium Ion That Has 10 Electrons Charge.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Ion That Has 10 Electrons Charge The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in. This electric charge generated on the ion is known. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. A magnesium ion therefore has 10 electrons. You can. Magnesium Ion That Has 10 Electrons Charge.
From ar.inspiredpencil.com
Magnesium Atom Structure Magnesium Ion That Has 10 Electrons Charge A magnesium ion therefore has 10 electrons. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. This electric charge generated on the ion is known. The symbol for the ion is mg 2+, and it is called a magnesium ion. 93 rows this table shows the most common charges for. Magnesium Ion That Has 10 Electrons Charge.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Magnesium Ion That Has 10 Electrons Charge 93 rows this table shows the most common charges for atoms of the chemical elements. This electric charge generated on the ion is known. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. You can use this table to predict whether an atom can bond with another atom. The first. Magnesium Ion That Has 10 Electrons Charge.
From www.pearson.com
Show the complete orbital diagram of magnesium. Channels for Pearson+ Magnesium Ion That Has 10 Electrons Charge The first shows common element charges, while the second shows all the element charges for the first 45 elements (most common charges in. 93 rows this table shows the most common charges for atoms of the chemical elements. You can use this table to predict whether an atom can bond with another atom. The symbol for the ion is mg. Magnesium Ion That Has 10 Electrons Charge.
From celbncbc.blob.core.windows.net
Magnesium Ion Number Of Electrons at Joan Harbert blog Magnesium Ion That Has 10 Electrons Charge For example, when a neutral sodium atom loses one electron, it becomes the sodium ion, na + as shown in the following shorthand notation: The charge on a magnesium ion with ten electrons would be +2, since magnesium typically has 12 electrons in its neutral state. This electric charge generated on the ion is known. The first shows common element. Magnesium Ion That Has 10 Electrons Charge.