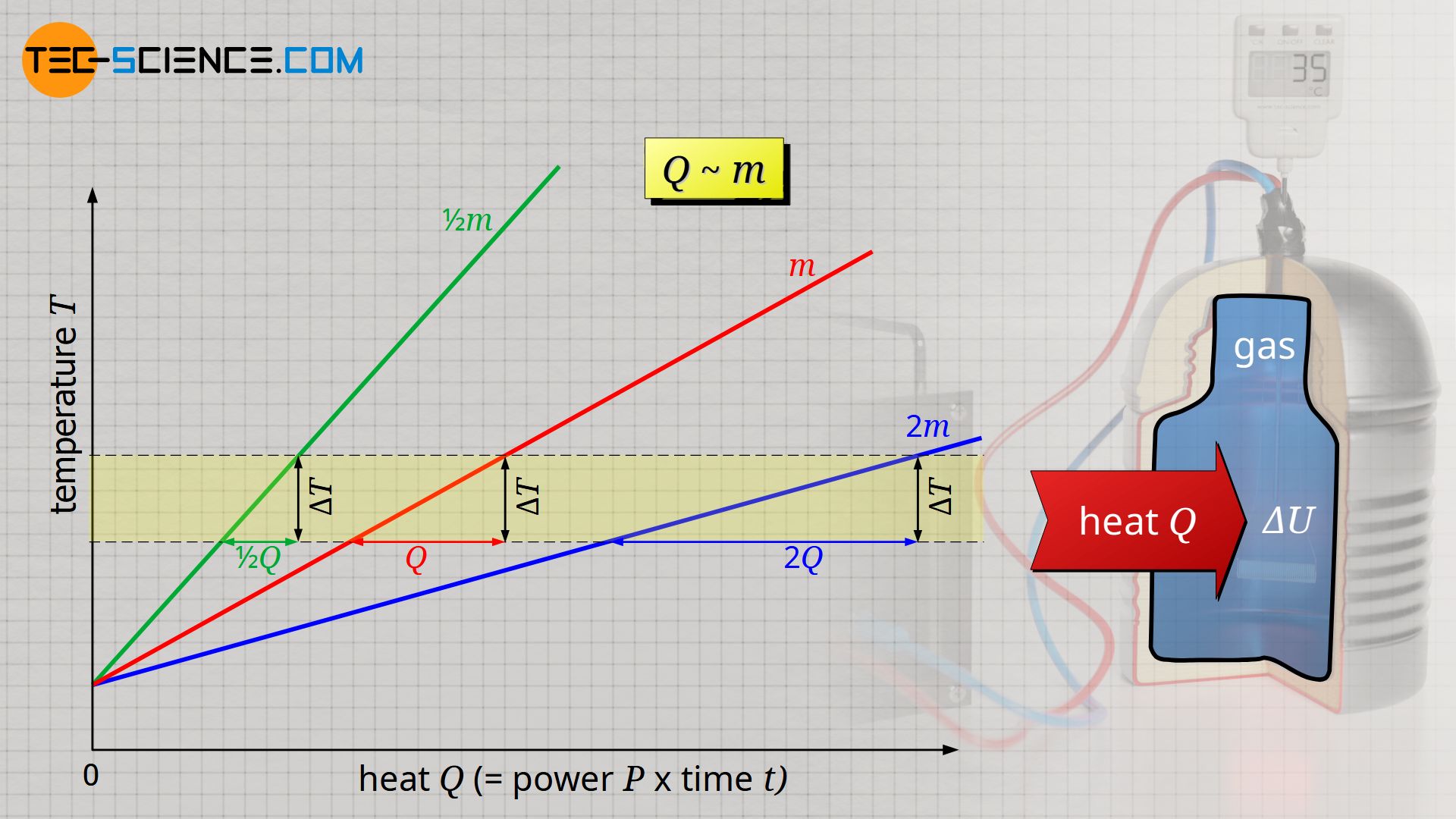
Thermodynamic Cycle Change In Internal Energy . The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Regardless of the shape of the cycle, whether it is reversible or not, the type of. The variation of internal energy in a cycle is always zero. The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. The first law of thermodynamics states that the. When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path is equal to zero. Δ u = q − w. Δ u = q − w. The 1 st law states that:
from www.tec-science.com
Regardless of the shape of the cycle, whether it is reversible or not, the type of. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Δ u = q − w. The first law of thermodynamics states that the. The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. The variation of internal energy in a cycle is always zero. When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path is equal to zero. The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: Δ u = q − w. The 1 st law states that:
Calculation of the internal energy for ideal gases tecscience
Thermodynamic Cycle Change In Internal Energy A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: The first law of thermodynamics states that the. Δ u = q − w. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path is equal to zero. The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. Δ u = q − w. The 1 st law states that: Regardless of the shape of the cycle, whether it is reversible or not, the type of. The variation of internal energy in a cycle is always zero.
From www.tec-science.com
Calculation of the internal energy for ideal gases tecscience Thermodynamic Cycle Change In Internal Energy Δ u = q − w. The variation of internal energy in a cycle is always zero. The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: Regardless of the shape of the cycle, whether it is reversible or not, the type of. The 1. Thermodynamic Cycle Change In Internal Energy.
From www.youtube.com
Thermodynamics 1 C2 L10 First law of thermodynamics Energy Thermodynamic Cycle Change In Internal Energy Δ u = q − w. The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. The 1 st law states that: Δ u = q − w. Regardless of the shape of the cycle, whether it is reversible or not, the type of. The change in the internal. Thermodynamic Cycle Change In Internal Energy.
From www.youtube.com
Internal Energy of Gas Thermodynamic Systems YouTube Thermodynamic Cycle Change In Internal Energy The 1 st law states that: The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. Δ u = q − w. The first law of thermodynamics states that the. Δ u = q − w. When a closed cycle is considered for the first law of thermodynamics, the. Thermodynamic Cycle Change In Internal Energy.
From mechaniclove.com
Thermodynamic Cycles Thermodynamic Cycle Change In Internal Energy The variation of internal energy in a cycle is always zero. The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Δ u = q − w. When a closed cycle is. Thermodynamic Cycle Change In Internal Energy.
From byjus.com
First Law of Thermodynamics Equations, Limitations and Examples Thermodynamic Cycle Change In Internal Energy The first law of thermodynamics states that the. The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. The variation of internal energy in. Thermodynamic Cycle Change In Internal Energy.
From www.youtube.com
Thermodynamic Processes Isobaric, Isochoric, Isothermal and Adiabatic Thermodynamic Cycle Change In Internal Energy Δ u = q − w. The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. Regardless of the shape of the cycle, whether. Thermodynamic Cycle Change In Internal Energy.
From www.slideshare.net
Thermodynamic cycles Thermodynamic Cycle Change In Internal Energy When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path is equal to zero. The first law of thermodynamics states that the. The 1 st law states that: The change in the internal energy of the system, δ u δ u, is related to heat and work by the. Thermodynamic Cycle Change In Internal Energy.
From www.numerade.com
SOLVEDAssertion In a complete thermodynamic cycle heat supplied to a Thermodynamic Cycle Change In Internal Energy The first law of thermodynamics states that the. When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path is equal to zero. Δ u = q − w. The change in the internal energy of the system, δ u δ u, is related to heat and work by the. Thermodynamic Cycle Change In Internal Energy.
From www.youtube.com
First Law of Thermodynamics, Basic Introduction Internal Energy, Heat Thermodynamic Cycle Change In Internal Energy The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. A reaction or process in which heat is transferred to a system from its. Thermodynamic Cycle Change In Internal Energy.
From www.doubtnut.com
The given pV diagram represents the thermodynamic cycle of an eng Thermodynamic Cycle Change In Internal Energy A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Δ u = q − w. The variation of internal energy in a cycle is always zero. The 1 st law states that: When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole. Thermodynamic Cycle Change In Internal Energy.
From www.youtube.com
Thermodynamics Internal Energy YouTube Thermodynamic Cycle Change In Internal Energy The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. The first law of thermodynamics states that the. When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path is equal to zero. Δ u = q − w.. Thermodynamic Cycle Change In Internal Energy.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Thermodynamic Cycle Change In Internal Energy A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: Δ u = q − w. The first law of thermodynamics is actually the law of conservation of. Thermodynamic Cycle Change In Internal Energy.
From www.youtube.com
02 THERMODYNAMIC PROCESS & CHANGE IN INTERNAL ENERGY YouTube Thermodynamic Cycle Change In Internal Energy The 1 st law states that: Regardless of the shape of the cycle, whether it is reversible or not, the type of. The first law of thermodynamics states that the. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Δ u = q − w. The variation of internal energy in a. Thermodynamic Cycle Change In Internal Energy.
From thermalengineeringlearn.blogspot.com
THERMAL ENGINEERING Thermodynamic Equilibrium Thermodynamic Cycle Change In Internal Energy The first law of thermodynamics states that the. The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: Δ u = q − w. When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path. Thermodynamic Cycle Change In Internal Energy.
From www.slideserve.com
PPT A gas undergoes a thermodynamic cycle consisting of three Thermodynamic Cycle Change In Internal Energy The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: The 1 st law states that: The variation of internal energy in a cycle is always zero. Regardless of the shape of the cycle, whether it is reversible or not, the type of. A reaction. Thermodynamic Cycle Change In Internal Energy.
From www.slideserve.com
PPT Thermodynamic Processes PowerPoint Presentation, free download Thermodynamic Cycle Change In Internal Energy The 1 st law states that: The first law of thermodynamics states that the. The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path. Thermodynamic Cycle Change In Internal Energy.
From www.chemistrylearner.com
First Law of Thermodynamics Statement, Equation, & Examples Thermodynamic Cycle Change In Internal Energy The variation of internal energy in a cycle is always zero. The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: Regardless of the shape of the cycle, whether it is reversible or not, the type of. A reaction or process in which heat is. Thermodynamic Cycle Change In Internal Energy.
From scienceinfo.com
Thermodynamic Processes Science Info Thermodynamic Cycle Change In Internal Energy The variation of internal energy in a cycle is always zero. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. The 1 st law states that: The change in the internal. Thermodynamic Cycle Change In Internal Energy.
From www.youtube.com
First Law of Thermodynamics Thermal energy and Work Done YouTube Thermodynamic Cycle Change In Internal Energy A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Δ u = q − w. The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: The variation of internal energy in a cycle is always zero. The. Thermodynamic Cycle Change In Internal Energy.
From www.youtube.com
internal energy thermodynamics INTERNAL ENERGY IS POINT FUNCTION Thermodynamic Cycle Change In Internal Energy The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. Δ u = q − w. When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path is equal to zero. Δ u = q − w. The variation. Thermodynamic Cycle Change In Internal Energy.
From www.researchgate.net
A thermodynamic cycle for a transcritical CO2 heat pump with a Thermodynamic Cycle Change In Internal Energy The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: The 1 st law states that: Δ u = q − w. The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. When a. Thermodynamic Cycle Change In Internal Energy.
From www.tec-science.com
Internal energy & first law of thermodynamics tecscience Thermodynamic Cycle Change In Internal Energy The variation of internal energy in a cycle is always zero. The 1 st law states that: The first law of thermodynamics states that the. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Δ u = q − w. The change in the internal energy of the system, δ u δ. Thermodynamic Cycle Change In Internal Energy.
From www.youtube.com
Isobaric Process Thermodynamics Work & Heat Energy, Molar Heat Thermodynamic Cycle Change In Internal Energy The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. Regardless of the shape of the cycle, whether it is reversible or not, the. Thermodynamic Cycle Change In Internal Energy.
From www.grc.nasa.gov
First Law of Thermodynamics Thermodynamic Cycle Change In Internal Energy The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path is equal to zero. The first law of thermodynamics states that the. The 1 st law states that:. Thermodynamic Cycle Change In Internal Energy.
From www.tec-science.com
Internal energy & first law of thermodynamics tecscience Thermodynamic Cycle Change In Internal Energy When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path is equal to zero. The variation of internal energy in a cycle is always zero. The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of. Thermodynamic Cycle Change In Internal Energy.
From courses.lumenlearning.com
The First Law of Thermodynamics Biology for Majors I Thermodynamic Cycle Change In Internal Energy The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful in thermodynamics. The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: When a closed cycle is considered for the first law of thermodynamics, the change. Thermodynamic Cycle Change In Internal Energy.
From www.doubtnut.com
Three processes compose a thermodynamic cycle shown in the Thermodynamic Cycle Change In Internal Energy Δ u = q − w. The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path is equal to zero. The 1 st law. Thermodynamic Cycle Change In Internal Energy.
From www.slideshare.net
Thermodynamic cycles Thermodynamic Cycle Change In Internal Energy Δ u = q − w. Regardless of the shape of the cycle, whether it is reversible or not, the type of. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path. Thermodynamic Cycle Change In Internal Energy.
From www.slideshare.net
Thermodynamic cycles Thermodynamic Cycle Change In Internal Energy The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: Δ u = q − w. The 1 st law states that: When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path is equal. Thermodynamic Cycle Change In Internal Energy.
From slideplayer.com
Thermodynamic Processes ppt download Thermodynamic Cycle Change In Internal Energy Regardless of the shape of the cycle, whether it is reversible or not, the type of. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Δ u = q − w. The first law of thermodynamics states that the. The variation of internal energy in a cycle is always zero. Δ u. Thermodynamic Cycle Change In Internal Energy.
From www.tes.com
GCSEInternal energy Teaching Resources Thermodynamic Cycle Change In Internal Energy The variation of internal energy in a cycle is always zero. Δ u = q − w. Regardless of the shape of the cycle, whether it is reversible or not, the type of. The first law of thermodynamics states that the. The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful. Thermodynamic Cycle Change In Internal Energy.
From www.youtube.com
The First Law of Thermodynamics Internal Energy, Heat, and Work YouTube Thermodynamic Cycle Change In Internal Energy When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path is equal to zero. Δ u = q − w. The first law of thermodynamics states that the. The variation of internal energy in a cycle is always zero. The 1 st law states that: Δ u = q. Thermodynamic Cycle Change In Internal Energy.
From www.studypool.com
SOLUTION Thermodynamic cycle and energy of thermodynamic system Thermodynamic Cycle Change In Internal Energy The first law of thermodynamics states that the. The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: A reaction or process in which heat is transferred to a system from its surroundings is endothermic. When a closed cycle is considered for the first law. Thermodynamic Cycle Change In Internal Energy.
From www.eigenplus.com
Thermodynamic Process Definition, Types & Examples eigenplus Thermodynamic Cycle Change In Internal Energy Regardless of the shape of the cycle, whether it is reversible or not, the type of. The change in the internal energy of the system, δ u δ u, is related to heat and work by the first law of thermodynamics: The first law of thermodynamics is actually the law of conservation of energy stated in a form most useful. Thermodynamic Cycle Change In Internal Energy.
From www.theengineeringconcepts.com
Thermodynamic Cycle Archives The Engineering Concepts Thermodynamic Cycle Change In Internal Energy When a closed cycle is considered for the first law of thermodynamics, the change in internal energy around the whole path is equal to zero. The 1 st law states that: The variation of internal energy in a cycle is always zero. The change in the internal energy of the system, δ u δ u, is related to heat and. Thermodynamic Cycle Change In Internal Energy.