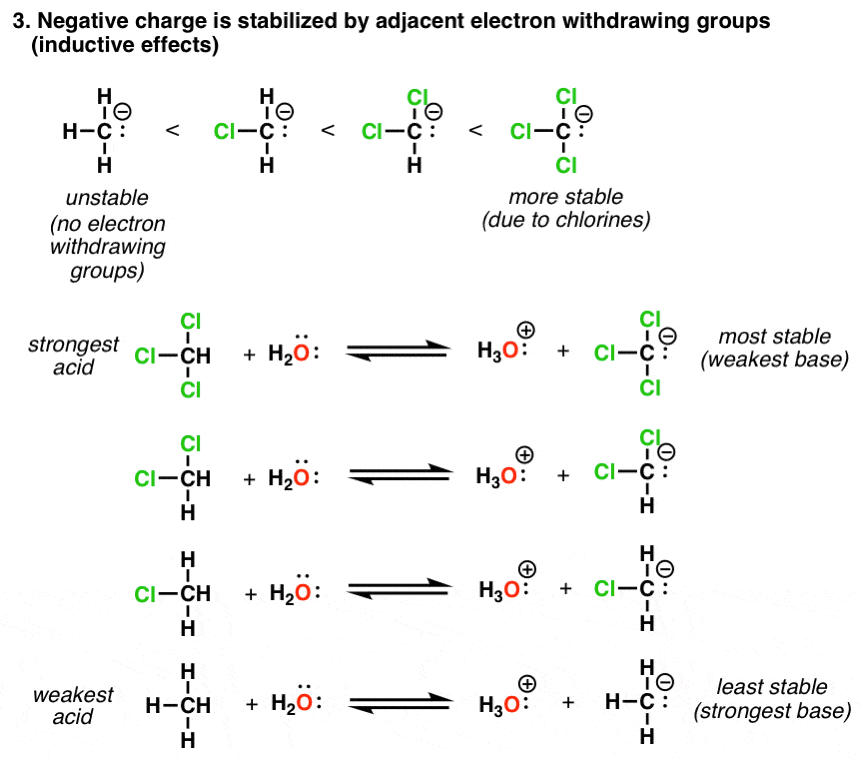
How Does Induction Affect Acidity . More chlorine atoms have an overall stronger. The inductive effect is addictive; The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to your orgo practice questions. Acidity trends in organic molecules. In addition, the inductive takes place through covalent bonds, and its influence decreases markedly with distance—thus a chlorine two carbons away from a carboxylic acid group has a. Organic chemistry tutorial video on the inductive effect on acids and bases. Here, we’ll review the key trends that affect acidity,. One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. Five key factors that influence acidity. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound.
from www.masterorganicchemistry.com
Here, we’ll review the key trends that affect acidity,. More chlorine atoms have an overall stronger. One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. In addition, the inductive takes place through covalent bonds, and its influence decreases markedly with distance—thus a chlorine two carbons away from a carboxylic acid group has a. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. The inductive effect is addictive; Organic chemistry tutorial video on the inductive effect on acids and bases. Five key factors that influence acidity. This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to your orgo practice questions. Acidity trends in organic molecules.
Acidity Trends In Organic Chemistry Master Organic Chemistry
How Does Induction Affect Acidity More chlorine atoms have an overall stronger. Five key factors that influence acidity. Acidity trends in organic molecules. More chlorine atoms have an overall stronger. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. Here, we’ll review the key trends that affect acidity,. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. Organic chemistry tutorial video on the inductive effect on acids and bases. In addition, the inductive takes place through covalent bonds, and its influence decreases markedly with distance—thus a chlorine two carbons away from a carboxylic acid group has a. The inductive effect is addictive; This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to your orgo practice questions.
From openpress.usask.ca
6.3. Qualitative Estimates of Acidity Introduction to Organic Chemistry How Does Induction Affect Acidity The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. Here, we’ll review the key trends that affect acidity,. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. In addition,. How Does Induction Affect Acidity.
From www.youtube.com
Inductive effect Acidity of compounds General Organic Chemistry YouTube How Does Induction Affect Acidity More chlorine atoms have an overall stronger. Here, we’ll review the key trends that affect acidity,. The inductive effect is addictive; Organic chemistry tutorial video on the inductive effect on acids and bases. Five key factors that influence acidity. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. One of the most important applications of. How Does Induction Affect Acidity.
From scienceinfo.com
Inductive Effect Types, Uses, Stability How Does Induction Affect Acidity Acidity trends in organic molecules. Five key factors that influence acidity. In addition, the inductive takes place through covalent bonds, and its influence decreases markedly with distance—thus a chlorine two carbons away from a carboxylic acid group has a. Here, we’ll review the key trends that affect acidity,. Inductive effects and charge delocalization significantly influence the acidity or basicity of. How Does Induction Affect Acidity.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID574436 How Does Induction Affect Acidity The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. Acidity trends in organic molecules. Five key factors that influence acidity. This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to your orgo practice questions. Inductive effects and charge delocalization significantly influence the acidity. How Does Induction Affect Acidity.
From www.youtube.com
Comparing Acidities Inductive Effects YouTube How Does Induction Affect Acidity More chlorine atoms have an overall stronger. The inductive effect is addictive; In addition, the inductive takes place through covalent bonds, and its influence decreases markedly with distance—thus a chlorine two carbons away from a carboxylic acid group has a. Here, we’ll review the key trends that affect acidity,. Acidity trends in organic molecules. One of the most important applications. How Does Induction Affect Acidity.
From chemizi.blogspot.com
Inductive effect and field effect in organic chemistry How Does Induction Affect Acidity The inductive effect is addictive; This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to your orgo practice questions. Five key factors that influence acidity. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. One of the most important applications of the inductive. How Does Induction Affect Acidity.
From www.masterorganicchemistry.com
5 Key Factors That Influence Acidity In Organic Chemistry How Does Induction Affect Acidity Here, we’ll review the key trends that affect acidity,. Organic chemistry tutorial video on the inductive effect on acids and bases. The inductive effect is addictive; One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. Five key factors that influence acidity. Acidity trends in organic molecules. More chlorine. How Does Induction Affect Acidity.
From www.chegg.com
Solved What effect do resonance and induction have on the How Does Induction Affect Acidity Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. Five key factors that influence acidity. In addition, the inductive takes place through covalent bonds, and its influence decreases markedly with distance—thus a chlorine two. How Does Induction Affect Acidity.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6708642 How Does Induction Affect Acidity More chlorine atoms have an overall stronger. Organic chemistry tutorial video on the inductive effect on acids and bases. Acidity trends in organic molecules. The inductive effect is addictive; The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. In addition, the inductive takes place through covalent bonds, and its influence decreases markedly with distance—thus a. How Does Induction Affect Acidity.
From www.youtube.com
Episode 4 Inductive Effects on Acidity YouTube How Does Induction Affect Acidity Organic chemistry tutorial video on the inductive effect on acids and bases. This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to your orgo practice questions. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. The inductive effect is addictive; One of the. How Does Induction Affect Acidity.
From www.slideserve.com
PPT FACTORS THAT INCREASE ACIDITY PowerPoint Presentation, free download ID2027692 How Does Induction Affect Acidity Five key factors that influence acidity. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. The inductive effect is addictive;. How Does Induction Affect Acidity.
From www.masterorganicchemistry.com
Acidity Trends In Organic Chemistry Master Organic Chemistry How Does Induction Affect Acidity The inductive effect is addictive; The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. Here, we’ll review the key trends that affect acidity,. Acidity trends in organic molecules. One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. This video gives you a detailed. How Does Induction Affect Acidity.
From www.youtube.com
What is Electron Withdrawing Groups ? Inductive effect & Acidity Basics One Chemistry How Does Induction Affect Acidity Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Organic chemistry tutorial video on the inductive effect on acids and bases. Acidity trends in organic molecules. More chlorine atoms have an overall stronger. This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to. How Does Induction Affect Acidity.
From www.masterorganicchemistry.com
5 Key Factors That Influence Acidity In Organic Chemistry How Does Induction Affect Acidity One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. More chlorine atoms have an overall stronger. Acidity trends in organic molecules. In addition, the inductive takes place through covalent bonds, and its influence decreases. How Does Induction Affect Acidity.
From www.slideserve.com
PPT AcidBase and DonorAcceptor Chemistry PowerPoint Presentation, free download ID6639815 How Does Induction Affect Acidity In addition, the inductive takes place through covalent bonds, and its influence decreases markedly with distance—thus a chlorine two carbons away from a carboxylic acid group has a. Here, we’ll review the key trends that affect acidity,. Five key factors that influence acidity. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. One of the. How Does Induction Affect Acidity.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6708642 How Does Induction Affect Acidity More chlorine atoms have an overall stronger. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. The inductive effect is addictive; This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply. How Does Induction Affect Acidity.
From byjus.com
Inductive Effect Types of Inductive Effect, Applications, Stability How Does Induction Affect Acidity This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to your orgo practice questions. More chlorine atoms have an overall stronger. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. Acidity trends in organic molecules. The inductive effect is addictive; One of the. How Does Induction Affect Acidity.
From www.youtube.com
Inductive effectRelative AcidityBasic chemistryPart 2NJCHEMISTRYI, +I How Does Induction Affect Acidity One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. Five key factors that influence acidity. Acidity trends in organic molecules. Here, we’ll review the key trends that affect acidity,. More chlorine atoms have an overall stronger. This video gives you a detailed explanation of induction and walks you. How Does Induction Affect Acidity.
From www.youtube.com
Inductive Effect Reaction Mechanisms YouTube How Does Induction Affect Acidity The inductive effect is addictive; Five key factors that influence acidity. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Acidity trends in organic molecules. Organic chemistry tutorial video on the inductive effect on acids and bases. In addition, the inductive takes place through covalent bonds, and its influence decreases markedly with distance—thus a chlorine. How Does Induction Affect Acidity.
From www.youtube.com
Induction vs Resonance (Rules of Organic Chemistry 4) YouTube How Does Induction Affect Acidity This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to your orgo practice questions. Here, we’ll review the key trends that affect acidity,. The inductive effect is addictive; The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. Acidity trends in organic molecules. More. How Does Induction Affect Acidity.
From openpress.usask.ca
6.3. Qualitative Estimates of Acidity Introduction to Organic Chemistry How Does Induction Affect Acidity In addition, the inductive takes place through covalent bonds, and its influence decreases markedly with distance—thus a chlorine two carbons away from a carboxylic acid group has a. Acidity trends in organic molecules. Organic chemistry tutorial video on the inductive effect on acids and bases. One of the most important applications of the inductive effect in organic chemistry relates to. How Does Induction Affect Acidity.
From leah4sci.com
Inductive Effect on Acid Base Strength in Organic Chemistry How Does Induction Affect Acidity The inductive effect is addictive; In addition, the inductive takes place through covalent bonds, and its influence decreases markedly with distance—thus a chlorine two carbons away from a carboxylic acid group has a. This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to your orgo practice questions. Acidity. How Does Induction Affect Acidity.
From chemistnotes.com
Inductive Effect Definitions/ Examples Chemistry Notes How Does Induction Affect Acidity One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. Acidity trends in organic molecules. In addition, the inductive takes place through covalent bonds, and its influence decreases markedly with distance—thus a chlorine two carbons away from a carboxylic acid group has a. Here, we’ll review the key trends. How Does Induction Affect Acidity.
From www.masterorganicchemistry.com
5 Key Factors That Influence Acidity In Organic Chemistry How Does Induction Affect Acidity The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. More chlorine atoms have an overall stronger. Organic chemistry tutorial video on the inductive effect on acids and bases. In addition, the inductive takes place through covalent bonds, and its influence decreases markedly with distance—thus a chlorine two carbons away from a carboxylic acid group has. How Does Induction Affect Acidity.
From www.youtube.com
Acidity of Aromatic Hydrocarbons YouTube How Does Induction Affect Acidity More chlorine atoms have an overall stronger. One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to your orgo practice questions. In addition, the inductive takes place. How Does Induction Affect Acidity.
From www.chemistrysteps.com
Inductive and Resonance (Mesomeric) Effects Chemistry Steps How Does Induction Affect Acidity One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. More chlorine atoms have an overall stronger. Acidity trends in organic molecules. Here, we’ll review the key trends that affect acidity,. The inductive effect is addictive; Inductive effects and charge delocalization significantly influence the acidity or basicity of a. How Does Induction Affect Acidity.
From www.masterorganicchemistry.com
Acidity Trends In Organic Chemistry Master Organic Chemistry How Does Induction Affect Acidity More chlorine atoms have an overall stronger. Acidity trends in organic molecules. In addition, the inductive takes place through covalent bonds, and its influence decreases markedly with distance—thus a chlorine two carbons away from a carboxylic acid group has a. Here, we’ll review the key trends that affect acidity,. Inductive effects and charge delocalization significantly influence the acidity or basicity. How Does Induction Affect Acidity.
From www.slideserve.com
PPT Organic Acids and Bases PowerPoint Presentation, free download ID2883807 How Does Induction Affect Acidity Five key factors that influence acidity. More chlorine atoms have an overall stronger. This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to your orgo practice questions. In addition, the inductive takes place through covalent bonds, and its influence decreases markedly with distance—thus a chlorine two carbons away. How Does Induction Affect Acidity.
From www.slideserve.com
PPT Acid/Base in Organic Chemistry PowerPoint Presentation, free download ID9429313 How Does Induction Affect Acidity This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to your orgo practice questions. More chlorine atoms have an overall stronger. Acidity trends in organic molecules. The inductive effect is addictive; Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Five key factors. How Does Induction Affect Acidity.
From chem.libretexts.org
3.4 Structural Effects on Acidity and Basicity Chemistry LibreTexts How Does Induction Affect Acidity Here, we’ll review the key trends that affect acidity,. Organic chemistry tutorial video on the inductive effect on acids and bases. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. The inductive effect is. How Does Induction Affect Acidity.
From www.youtube.com
Inductive effect, acidity, basicity order.. YouTube How Does Induction Affect Acidity Five key factors that influence acidity. Here, we’ll review the key trends that affect acidity,. This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to your orgo practice questions. The inductive effect is addictive; The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds.. How Does Induction Affect Acidity.
From dpuhospital.com
Causes of Acidity Comprehensive Overview DPU Hospital How Does Induction Affect Acidity One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Here, we’ll review the key trends that affect acidity,. The inductive effect is addictive; More chlorine atoms have an overall stronger. In addition, the inductive. How Does Induction Affect Acidity.
From www.youtube.com
What is Inductive Effect? Application in stability of reaction intermediates & acidity and How Does Induction Affect Acidity This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to your orgo practice questions. Acidity trends in organic molecules. Here, we’ll review the key trends that affect acidity,. Organic chemistry tutorial video on the inductive effect on acids and bases. Inductive effects and charge delocalization significantly influence the. How Does Induction Affect Acidity.
From www.masterorganicchemistry.com
Acidity Trends In Organic Chemistry Master Organic Chemistry How Does Induction Affect Acidity This video gives you a detailed explanation of induction and walks you through practice questions to ensure you can apply it to your orgo practice questions. Organic chemistry tutorial video on the inductive effect on acids and bases. One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. Inductive. How Does Induction Affect Acidity.
From www.youtube.com
Inductive effectpositive and negative inductive effectAcidity and basicity with examples YouTube How Does Induction Affect Acidity Acidity trends in organic molecules. More chlorine atoms have an overall stronger. One of the most important applications of the inductive effect in organic chemistry relates to the acidity and basicity of molecules. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. In addition, the inductive takes place through covalent bonds, and its influence decreases. How Does Induction Affect Acidity.