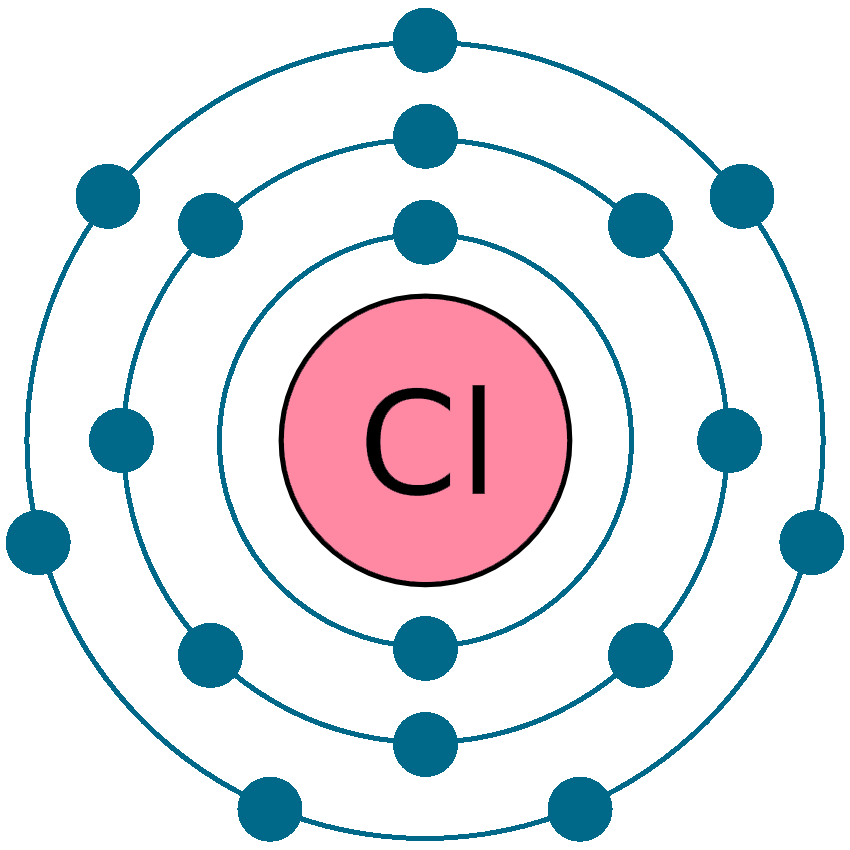
Chlorine Electron Dot . Chlorine has an atomic number of 17, meaning it has 17. Chlorine has seven valence electrons, so its lewis dot diagram consists of a symbol for chlorine surrounded by seven dots representing these. The electron configuration for chlorine could be written as: On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. The lewis structure indicates that. The core electrons would be 1 s2 2 s2 2 p6. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: Therefore it has seven valence electrons and needs to have seven dots drawn around its. 1 s2 2 s2 2 p6 3 s2 3 p5. Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures below. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of.
from www.newtondesk.com
1 s2 2 s2 2 p6 3 s2 3 p5. Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures below. Chlorine has an atomic number of 17, meaning it has 17. The lewis structure indicates that. On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: The electron configuration for chlorine could be written as: Chlorine has seven valence electrons, so its lewis dot diagram consists of a symbol for chlorine surrounded by seven dots representing these. The core electrons would be 1 s2 2 s2 2 p6.
Chlorine Cl (Element 17) of Periodic Table Newton Desk
Chlorine Electron Dot The lewis structure indicates that. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: The lewis structure indicates that. Chlorine has an atomic number of 17, meaning it has 17. On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. 1 s2 2 s2 2 p6 3 s2 3 p5. Therefore it has seven valence electrons and needs to have seven dots drawn around its. The electron configuration for chlorine could be written as: Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures below. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of. The core electrons would be 1 s2 2 s2 2 p6. Chlorine has seven valence electrons, so its lewis dot diagram consists of a symbol for chlorine surrounded by seven dots representing these.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Electron Dot The core electrons would be 1 s2 2 s2 2 p6. The electron configuration for chlorine could be written as: For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures. Chlorine Electron Dot.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Electron Dot Therefore it has seven valence electrons and needs to have seven dots drawn around its. Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures below. The lewis structure indicates that. The electron configuration for chlorine could be written as: Chlorine has an atomic number of. Chlorine Electron Dot.
From manualdatafootrest.z14.web.core.windows.net
Chlorine Electron Dot Diagram Chlorine Electron Dot The lewis structure indicates that. Chlorine has an atomic number of 17, meaning it has 17. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: Chlorine has seven valence electrons, so its lewis dot diagram consists of a symbol for chlorine surrounded by seven dots representing these. The core electrons would be 1. Chlorine Electron Dot.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Electron Dot A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of. On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. Therefore it has seven valence electrons and needs to have seven dots drawn around its. 1 s2 2 s2. Chlorine Electron Dot.
From www.thesciencehive.co.uk
Bonding and Structure* — the science sauce Chlorine Electron Dot Chlorine has an atomic number of 17, meaning it has 17. Chlorine has seven valence electrons, so its lewis dot diagram consists of a symbol for chlorine surrounded by seven dots representing these. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: 1 s2 2 s2 2 p6 3 s2 3 p5. The. Chlorine Electron Dot.
From wiringguidefrosts.z19.web.core.windows.net
Chlorine Dot And Cross Diagram Chlorine Electron Dot 1 s2 2 s2 2 p6 3 s2 3 p5. Chlorine has seven valence electrons, so its lewis dot diagram consists of a symbol for chlorine surrounded by seven dots representing these. The lewis structure indicates that. On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. Chlorine has an atomic number of 17,. Chlorine Electron Dot.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Electron Dot Therefore it has seven valence electrons and needs to have seven dots drawn around its. The lewis structure indicates that. Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures below. For example, when two chlorine atoms form a chlorine molecule, they share one pair of. Chlorine Electron Dot.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Electron Dot A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of. The electron configuration for chlorine could be written as: Chlorine has seven valence electrons, so its lewis dot diagram consists of a symbol for chlorine surrounded by seven dots representing these. Chlorine has an atomic. Chlorine Electron Dot.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Electron Dot Chlorine has an atomic number of 17, meaning it has 17. Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures below. The lewis structure indicates that. Therefore it has seven valence electrons and needs to have seven dots drawn around its. 1 s2 2 s2. Chlorine Electron Dot.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Chlorine Electron Dot The core electrons would be 1 s2 2 s2 2 p6. The lewis structure indicates that. The electron configuration for chlorine could be written as: A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of. Chlorine has seven valence electrons, so its lewis dot diagram. Chlorine Electron Dot.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Electron Dot 1 s2 2 s2 2 p6 3 s2 3 p5. The lewis structure indicates that. The electron configuration for chlorine could be written as: For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. Chlorine has seven valence. Chlorine Electron Dot.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Electron Dot The lewis structure indicates that. Therefore it has seven valence electrons and needs to have seven dots drawn around its. Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures below. For example, when two chlorine atoms form a chlorine molecule, they share one pair of. Chlorine Electron Dot.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Electron Dot The lewis structure indicates that. Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures below. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of. Chlorine has an atomic number. Chlorine Electron Dot.
From pnghero.com
Dot Formula Lewis Structure Chlorine Chloride Electron Diagram PNG Chlorine Electron Dot Chlorine has an atomic number of 17, meaning it has 17. On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. 1 s2 2 s2 2 p6 3 s2 3 p5. Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot. Chlorine Electron Dot.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Chlorine Electron Dot Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures below. The lewis structure indicates that. On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. 1 s2 2 s2 2 p6 3 s2 3 p5. A lewis electron dot. Chlorine Electron Dot.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Electron Dot The electron configuration for chlorine could be written as: For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: The lewis structure indicates that. Chlorine has an atomic number of 17, meaning it has 17. Chlorine has seven valence electrons, so its lewis dot diagram consists of a symbol for chlorine surrounded by seven. Chlorine Electron Dot.
From www.shutterstock.com
585 Chlorine electrons 이미지, 스톡 사진 및 벡터 Shutterstock Chlorine Electron Dot Chlorine has an atomic number of 17, meaning it has 17. On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. Therefore it has seven valence electrons and needs to have seven dots drawn around its. 1 s2 2 s2 2 p6 3 s2 3 p5. Figure \(\pageindex{1}\) the figure above shows the electron. Chlorine Electron Dot.
From manualfixdercombustion.z13.web.core.windows.net
Chlorine Electron Dot Diagram Chlorine Electron Dot Chlorine has an atomic number of 17, meaning it has 17. On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. The electron configuration for chlorine could be written as: A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons. Chlorine Electron Dot.
From exatin.info
Electron Dot Diagram For Chlorine exatin.info Chlorine Electron Dot Chlorine has an atomic number of 17, meaning it has 17. The lewis structure indicates that. The core electrons would be 1 s2 2 s2 2 p6. On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. 1 s2 2 s2 2 p6 3 s2 3 p5. Chlorine has seven valence electrons, so its. Chlorine Electron Dot.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Electron Dot On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. Therefore it has seven valence electrons and needs to have seven dots drawn around its. The lewis structure indicates that. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons. Chlorine Electron Dot.
From exatin.info
Electron Dot Diagram For Chlorine exatin.info Chlorine Electron Dot A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of. Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures below. The core electrons would be 1 s2 2 s2 2. Chlorine Electron Dot.
From chemtech-us.com
15 Interesting Facts About Chlorine Chlorine Electron Dot 1 s2 2 s2 2 p6 3 s2 3 p5. Chlorine has seven valence electrons, so its lewis dot diagram consists of a symbol for chlorine surrounded by seven dots representing these. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: The lewis structure indicates that. On the periodic table, chlorine (cl) is. Chlorine Electron Dot.
From mageechemistry11.blogspot.com
Chemistry 11 Bohr and Lewis electron dot diagrams Chlorine Electron Dot A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of. The electron configuration for chlorine could be written as: For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: The core electrons would be 1 s2 2 s2 2. Chlorine Electron Dot.
From imgbin.com
Lewis Structure Electron Chlorine Diagram Chloride PNG, Clipart, Black Chlorine Electron Dot Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures below. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of. The core electrons would be 1 s2 2 s2 2. Chlorine Electron Dot.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Chlorine Electron Dot The electron configuration for chlorine could be written as: The lewis structure indicates that. Chlorine has seven valence electrons, so its lewis dot diagram consists of a symbol for chlorine surrounded by seven dots representing these. Chlorine has an atomic number of 17, meaning it has 17. Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl. Chlorine Electron Dot.
From enginelistchester.z5.web.core.windows.net
Chlorine Electron Dot Diagram Chlorine Electron Dot Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures below. 1 s2 2 s2 2 p6 3 s2 3 p5. Chlorine has an atomic number of 17, meaning it has 17. The electron configuration for chlorine could be written as: For example, when two chlorine. Chlorine Electron Dot.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Electron Dot On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. Therefore it has seven valence electrons and needs to have seven dots drawn around its. Chlorine has seven valence electrons, so its lewis dot diagram consists of a symbol for chlorine surrounded by seven dots representing these. The electron configuration for chlorine could be. Chlorine Electron Dot.
From sciencenotes.org
Chlorine Facts Chlorine Electron Dot Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures below. The core electrons would be 1 s2 2 s2 2 p6. Chlorine has seven valence electrons, so its lewis dot diagram consists of a symbol for chlorine surrounded by seven dots representing these. For example,. Chlorine Electron Dot.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Electron Dot The core electrons would be 1 s2 2 s2 2 p6. Chlorine has an atomic number of 17, meaning it has 17. Therefore it has seven valence electrons and needs to have seven dots drawn around its. On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. A lewis electron dot diagram (or electron. Chlorine Electron Dot.
From www.youtube.com
How to Draw the Lewis Dot Structure for Cl (Chloride ion) YouTube Chlorine Electron Dot Chlorine has an atomic number of 17, meaning it has 17. Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures below. Therefore it has seven valence electrons and needs to have seven dots drawn around its. The core electrons would be 1 s2 2 s2. Chlorine Electron Dot.
From www.shutterstock.com
Periodic Simbol Chlorine Electron Structure Stock Vector (Royalty Free Chlorine Electron Dot Therefore it has seven valence electrons and needs to have seven dots drawn around its. Chlorine has seven valence electrons, so its lewis dot diagram consists of a symbol for chlorine surrounded by seven dots representing these. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence. Chlorine Electron Dot.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Electron Dot On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. Figure \(\pageindex{1}\) the figure above shows the electron shells of he (helium), cl (chlorine), and k (potassium) as well as their lewis dot structures below. 1 s2 2 s2 2 p6 3 s2 3 p5. The lewis structure indicates that. Chlorine has seven valence. Chlorine Electron Dot.
From www.dreamstime.com
Diagram Representation Element Chlorine Stock Illustrations 1 Diagram Chlorine Electron Dot Chlorine has an atomic number of 17, meaning it has 17. Therefore it has seven valence electrons and needs to have seven dots drawn around its. Chlorine has seven valence electrons, so its lewis dot diagram consists of a symbol for chlorine surrounded by seven dots representing these. A lewis electron dot diagram (or electron dot diagram, or a lewis. Chlorine Electron Dot.
From guidelibauditioned.z13.web.core.windows.net
Electron Dot Diagram For Chlorine Chlorine Electron Dot A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: Therefore it has seven valence electrons and needs to have seven dots drawn around its. Chlorine has an atomic. Chlorine Electron Dot.
From www.alamy.com
Bond formation in chlorine molecule. Illustration of the sharing of Chlorine Electron Dot A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of. On the periodic table, chlorine (cl) is located in group 17, also known as the halogens. The core electrons would be 1 s2 2 s2 2 p6. The electron configuration for chlorine could be written. Chlorine Electron Dot.