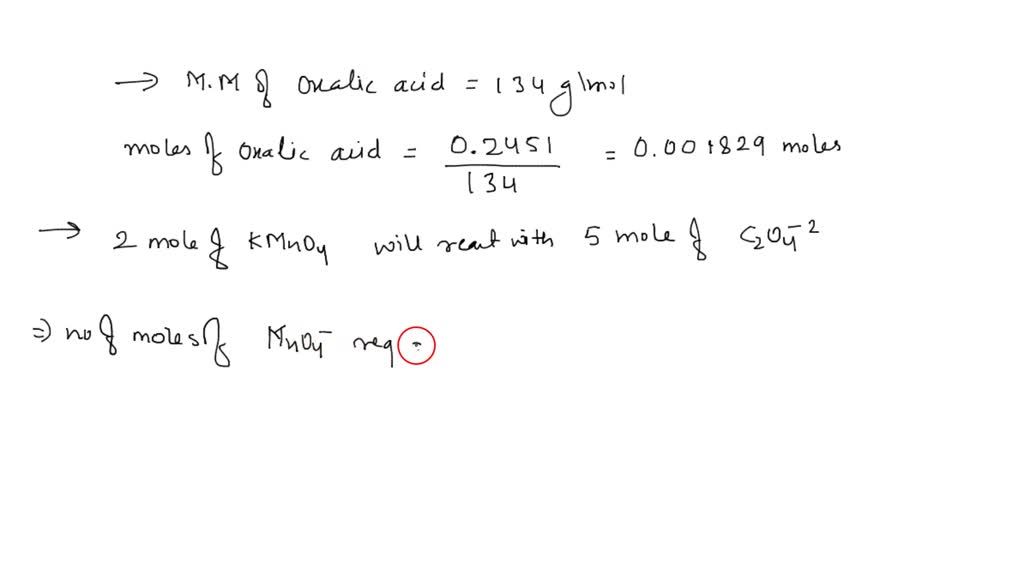Potassium Permanganate Titration End Point . Transition metals are involved in carrying titrations with potassium permanganate because they can form complex ions with potassium. Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Learn how to use potassium permanganate (kmno4) to determine the concentration of iron (as fe2+) in an unknown solution. A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Follow the steps to prepare, titrate and calculate. Learn how to use standardized potassium permanganate solution to determine the iron content of an unknown sample by acidic redox. Because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the concentration.
from www.numerade.com
Transition metals are involved in carrying titrations with potassium permanganate because they can form complex ions with potassium. Because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the concentration. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Learn how to use standardized potassium permanganate solution to determine the iron content of an unknown sample by acidic redox. Learn how to use potassium permanganate (kmno4) to determine the concentration of iron (as fe2+) in an unknown solution. Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration. Follow the steps to prepare, titrate and calculate. A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most.
SOLVED Potassium permanganate reacts with sodium oxalate by the
Potassium Permanganate Titration End Point Learn how to use standardized potassium permanganate solution to determine the iron content of an unknown sample by acidic redox. Follow the steps to prepare, titrate and calculate. A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Learn how to use standardized potassium permanganate solution to determine the iron content of an unknown sample by acidic redox. Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration. Because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the concentration. Transition metals are involved in carrying titrations with potassium permanganate because they can form complex ions with potassium. Learn how to use potassium permanganate (kmno4) to determine the concentration of iron (as fe2+) in an unknown solution. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration.
From exonshhzb.blob.core.windows.net
End Point Detection In Permanganate Titration at Marlene Ball blog Potassium Permanganate Titration End Point The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Transition metals are involved in carrying titrations with potassium permanganate because they can form complex ions with potassium. Learn how to use potassium permanganate (kmno4) to determine the concentration of iron (as fe2+) in an unknown solution. Follow. Potassium Permanganate Titration End Point.
From www.slideserve.com
PPT Objectives PowerPoint Presentation, free download ID2145720 Potassium Permanganate Titration End Point Transition metals are involved in carrying titrations with potassium permanganate because they can form complex ions with potassium. A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Follow the steps to prepare, titrate and calculate. Potassium permanganate acts as its own indicator, as the purple potassium. Potassium Permanganate Titration End Point.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Potassium Permanganate Titration End Point Learn how to use standardized potassium permanganate solution to determine the iron content of an unknown sample by acidic redox. A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Transition metals are involved in carrying titrations with potassium permanganate because they can form complex ions with. Potassium Permanganate Titration End Point.
From www.studocu.com
The titration of potassium permanganate In close proximity to the Potassium Permanganate Titration End Point Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration. Learn how to use standardized potassium permanganate solution to determine the iron content of an unknown sample by acidic redox. Learn how to use potassium permanganate (kmno4) to determine the concentration of iron (as fe2+) in an unknown solution. The titration of. Potassium Permanganate Titration End Point.
From www.alamy.com
Reading the level of potassium permanganate in a burette. When Potassium Permanganate Titration End Point Because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the concentration. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Learn how to use standardized potassium permanganate solution to determine the iron content of an. Potassium Permanganate Titration End Point.
From www.slideshare.net
Potassium permanganate titrations Potassium Permanganate Titration End Point Transition metals are involved in carrying titrations with potassium permanganate because they can form complex ions with potassium. Because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the concentration. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example. Potassium Permanganate Titration End Point.
From www.slideserve.com
PPT Potassium permanganate Titrations PowerPoint Presentation, free Potassium Permanganate Titration End Point A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Learn how to use standardized potassium permanganate solution to determine the iron content of an unknown sample by acidic redox. Transition metals are involved in carrying titrations with potassium permanganate because they can form complex ions with. Potassium Permanganate Titration End Point.
From www.coursehero.com
[Solved] A potassium permanganate solution with an approximate molarity Potassium Permanganate Titration End Point The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Learn how to use standardized potassium permanganate solution to determine the iron content of an unknown sample by acidic redox. Transition metals are involved in carrying titrations with potassium permanganate because they can form complex ions with potassium.. Potassium Permanganate Titration End Point.
From praxilabs.com
Standardization of Potassium Permanganate In 7 Steps Potassium Permanganate Titration End Point Follow the steps to prepare, titrate and calculate. A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Learn how to use standardized potassium permanganate solution to determine the iron content of an unknown sample by acidic redox. The titration of potassium permanganate (kmno 4) against oxalic. Potassium Permanganate Titration End Point.
From slideplayer.com
Titration Colour Changes ppt download Potassium Permanganate Titration End Point A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration. Because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant. Potassium Permanganate Titration End Point.
From exonshhzb.blob.core.windows.net
End Point Detection In Permanganate Titration at Marlene Ball blog Potassium Permanganate Titration End Point A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Learn how to use potassium permanganate (kmno4) to determine the concentration of iron (as fe2+) in an unknown solution. Follow the steps to prepare, titrate and calculate. The titration of potassium permanganate (kmno 4) against oxalic acid. Potassium Permanganate Titration End Point.
From www.sciencephoto.com
Potassium permanganate titration Stock Image C029/5908 Science Potassium Permanganate Titration End Point Learn how to use standardized potassium permanganate solution to determine the iron content of an unknown sample by acidic redox. Follow the steps to prepare, titrate and calculate. Because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the concentration. A useful property of a potassium permanganate solution is. Potassium Permanganate Titration End Point.
From www.numerade.com
SOLVEDPotassium iodide and potassiur permanganate solutions react as Potassium Permanganate Titration End Point Follow the steps to prepare, titrate and calculate. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Learn how to use potassium permanganate (kmno4) to determine the concentration of iron (as fe2+) in an unknown solution. Learn how to use standardized potassium permanganate solution to determine the. Potassium Permanganate Titration End Point.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Potassium Permanganate Titration End Point Transition metals are involved in carrying titrations with potassium permanganate because they can form complex ions with potassium. Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration. Learn how to use potassium permanganate (kmno4) to determine the concentration of iron (as fe2+) in an unknown solution. A useful property of a. Potassium Permanganate Titration End Point.
From www.scribd.com
Potassium Permanganate Titrations PDF Titration Chemistry Potassium Permanganate Titration End Point A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Follow the steps to prepare, titrate and calculate. Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration. Because this reaction is rapid and goes to completion, potassium permanganate. Potassium Permanganate Titration End Point.
From pixels.com
Potassium Permanganate Titration Photograph by Martyn F. Chillmaid Potassium Permanganate Titration End Point Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration. Because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the concentration. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example. Potassium Permanganate Titration End Point.
From www.numerade.com
SOLVED Potassium permanganate reacts with sodium oxalate by the Potassium Permanganate Titration End Point Learn how to use standardized potassium permanganate solution to determine the iron content of an unknown sample by acidic redox. Follow the steps to prepare, titrate and calculate. Because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the concentration. The titration of potassium permanganate (kmno 4) against oxalic. Potassium Permanganate Titration End Point.
From exonshhzb.blob.core.windows.net
End Point Detection In Permanganate Titration at Marlene Ball blog Potassium Permanganate Titration End Point Follow the steps to prepare, titrate and calculate. Transition metals are involved in carrying titrations with potassium permanganate because they can form complex ions with potassium. Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration. Learn how to use potassium permanganate (kmno4) to determine the concentration of iron (as fe2+) in. Potassium Permanganate Titration End Point.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Potassium Permanganate Titration End Point The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Learn how to use standardized potassium permanganate solution to determine the iron content of an. Potassium Permanganate Titration End Point.
From fphoto.photoshelter.com
science chemistry titration potassium permanganate Fundamental Potassium Permanganate Titration End Point Learn how to use potassium permanganate (kmno4) to determine the concentration of iron (as fe2+) in an unknown solution. A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration.. Potassium Permanganate Titration End Point.
From fphoto.photoshelter.com
science chemistry titration potassium permanganate sodium dichromate Potassium Permanganate Titration End Point The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added. Potassium Permanganate Titration End Point.
From studylib.net
Redox Titration The Standardization of Potassium Permanganate Potassium Permanganate Titration End Point Learn how to use potassium permanganate (kmno4) to determine the concentration of iron (as fe2+) in an unknown solution. A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Transition metals are involved in carrying titrations with potassium permanganate because they can form complex ions with potassium.. Potassium Permanganate Titration End Point.
From www.numerade.com
SOLVED When 0.4009 g of sodium cyanide is dissolved in solution, it Potassium Permanganate Titration End Point Learn how to use standardized potassium permanganate solution to determine the iron content of an unknown sample by acidic redox. Follow the steps to prepare, titrate and calculate. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. A useful property of a potassium permanganate solution is its. Potassium Permanganate Titration End Point.
From exonshhzb.blob.core.windows.net
End Point Detection In Permanganate Titration at Marlene Ball blog Potassium Permanganate Titration End Point Follow the steps to prepare, titrate and calculate. Learn how to use potassium permanganate (kmno4) to determine the concentration of iron (as fe2+) in an unknown solution. A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. The titration of potassium permanganate (kmno 4) against oxalic acid. Potassium Permanganate Titration End Point.
From wyattctxz.blogspot.com
Reaction Between Oxalic Acid And Potassium Permanganate Equation Potassium Permanganate Titration End Point Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration. Learn how to use potassium permanganate (kmno4) to determine the concentration of iron (as fe2+) in an unknown solution. Because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the concentration.. Potassium Permanganate Titration End Point.
From www.sciencephoto.com
Titration of potassium manganate (VII) Stock Image C030/3775 Potassium Permanganate Titration End Point The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Follow the steps to prepare, titrate and calculate. Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration. A useful property of a potassium permanganate solution is its intense purple. Potassium Permanganate Titration End Point.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记8.2.1 Redox Titration翰林国际教育 Potassium Permanganate Titration End Point Because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the concentration. Learn how to use potassium permanganate (kmno4) to determine the concentration of iron (as fe2+) in an unknown solution. Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration.. Potassium Permanganate Titration End Point.
From pixels.com
Potassium Permanganate Titration Photograph by Martyn F. Chillmaid Potassium Permanganate Titration End Point Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Transition metals are involved in carrying titrations with potassium permanganate because they can form complex ions with potassium. Learn how. Potassium Permanganate Titration End Point.
From www.alamy.com
Titration. Potassium permanganate (purple) being added, from a burette Potassium Permanganate Titration End Point The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Learn how to use potassium permanganate (kmno4) to determine the concentration of iron (as fe2+) in an unknown solution. Follow the steps to prepare, titrate and calculate. Transition metals are involved in carrying titrations with potassium permanganate because. Potassium Permanganate Titration End Point.
From www.slideserve.com
PPT Potassium permanganate Titrations PowerPoint Presentation, free Potassium Permanganate Titration End Point Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration. A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant. Potassium Permanganate Titration End Point.
From www.studypool.com
SOLUTION Preparation and standardization of potassium permanganate and Potassium Permanganate Titration End Point The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Learn how to use standardized potassium permanganate solution to determine the iron content of an unknown sample by acidic redox. A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve. Potassium Permanganate Titration End Point.
From www.slideshare.net
Potassium permanganate titrations Potassium Permanganate Titration End Point Because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the concentration. Follow the steps to prepare, titrate and calculate. Transition metals are involved in carrying titrations with potassium permanganate because they can form complex ions with potassium. Potassium permanganate acts as its own indicator, as the purple potassium. Potassium Permanganate Titration End Point.
From fphoto.photoshelter.com
science chemistry titration potassium permanganate Fundamental Potassium Permanganate Titration End Point A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Because this reaction is rapid and goes to completion, potassium permanganate (kmno 4) is widely used as a reactant for determining the concentration. Learn how to use standardized potassium permanganate solution to determine the iron content of. Potassium Permanganate Titration End Point.
From flatworldknowledge.lardbucket.org
Quantitative Analysis Using Titrations Potassium Permanganate Titration End Point The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration. Transition metals are involved in carrying titrations with potassium permanganate because they can form complex ions with potassium. A useful. Potassium Permanganate Titration End Point.
From www.chegg.com
Solved 8. Oxalic acid concentration may be determined in a Potassium Permanganate Titration End Point A useful property of a potassium permanganate solution is its intense purple color, which is sufficient to serve as an indicator for most. Learn how to use standardized potassium permanganate solution to determine the iron content of an unknown sample by acidic redox. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is. Potassium Permanganate Titration End Point.