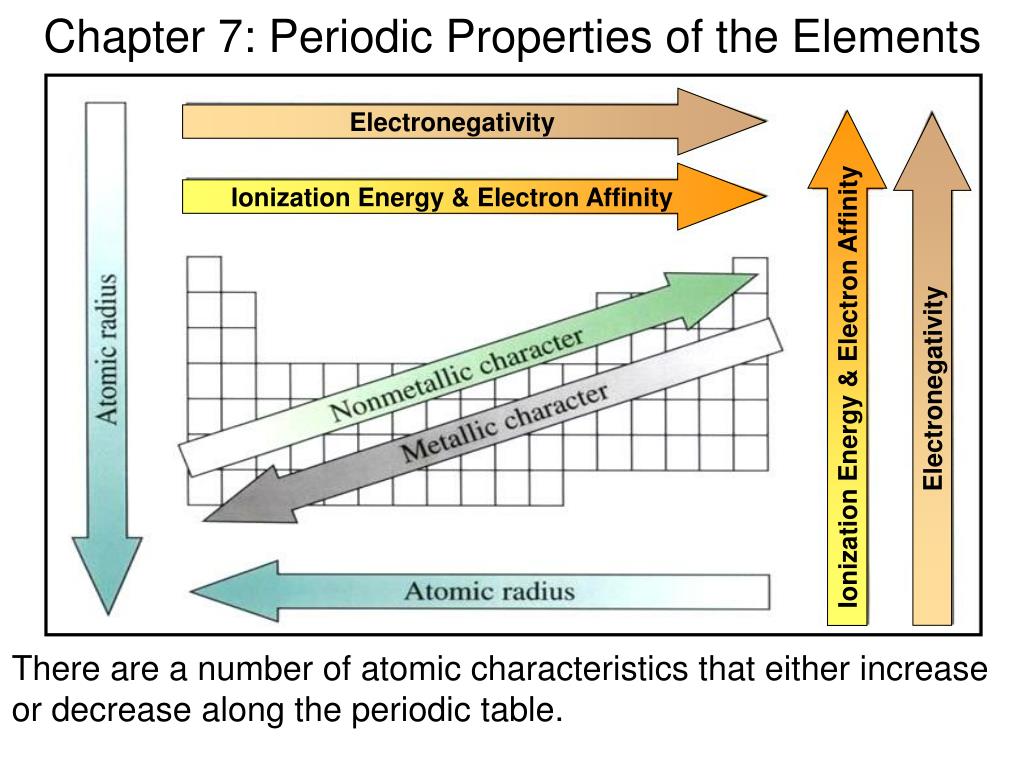
What Are The Properties Of Metals On The Periodic Table . Metals are solids at room temperature with the. Metals are located on the left side and the middle of the periodic table. The transition elements, groups ib. The majority of elements are metals and they are found on the left and in the. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Group ia and group iia (the alkali metals) are the most active metals. The position of an element on the periodic table provides information about its properties. Most of the elements are metals. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. Metals are solids at room temperature with the. Metals are lustrous, malleable, ductile, good conductors of heat and electricity.
from www.slideserve.com
Group ia and group iia (the alkali metals) are the most active metals. Most of the elements are metals. The transition elements, groups ib. The majority of elements are metals and they are found on the left and in the. The position of an element on the periodic table provides information about its properties. Metals are located on the left side and the middle of the periodic table. Metals are solids at room temperature with the. Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. Metals are solids at room temperature with the. Metals are lustrous, malleable, ductile, good conductors of heat and electricity.
PPT Chapter 7 Periodic Properties of the Elements PowerPoint
What Are The Properties Of Metals On The Periodic Table The transition elements, groups ib. The position of an element on the periodic table provides information about its properties. Group ia and group iia (the alkali metals) are the most active metals. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Metals are solids at room temperature with the. The transition elements, groups ib. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Most of the elements are metals. Metals are solids at room temperature with the. Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. Metals are located on the left side and the middle of the periodic table. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. The majority of elements are metals and they are found on the left and in the.
From chemwiki.ucdavis.edu
Periodic Properties of the Elements Chemwiki What Are The Properties Of Metals On The Periodic Table Metals are solids at room temperature with the. Metals are solids at room temperature with the. The transition elements, groups ib. Metals are located on the left side and the middle of the periodic table. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Group ia and group iia (the alkali metals) are the most active metals. The. What Are The Properties Of Metals On The Periodic Table.
From safetyzik.weebly.com
Metals periodic table safetyzik What Are The Properties Of Metals On The Periodic Table Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. Most of the elements are metals. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metals include the alkali metal, alkaline earth, transition metal,. What Are The Properties Of Metals On The Periodic Table.
From theconversation.com
Understanding the periodic table through the lens of the volatile Group What Are The Properties Of Metals On The Periodic Table Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. The position of an element on the periodic table provides information about its properties. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metals are located on the left side and the middle of the periodic table. Most of the elements are metals.. What Are The Properties Of Metals On The Periodic Table.
From kids.britannica.com
chemical compound Students Britannica Kids Homework Help What Are The Properties Of Metals On The Periodic Table Metals are solids at room temperature with the. Metals are solids at room temperature with the. Group ia and group iia (the alkali metals) are the most active metals. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metals are located on the left side and the middle of the periodic table. The transition elements, groups ib. The. What Are The Properties Of Metals On The Periodic Table.
From www.slideserve.com
PPT Lecture No.7 The Periodic Table and Some Properties of The What Are The Properties Of Metals On The Periodic Table Metals are located on the left side and the middle of the periodic table. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Group ia and group iia (the alkali metals) are the most active metals. Metals are lustrous, malleable, ductile, good conductors. What Are The Properties Of Metals On The Periodic Table.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint What Are The Properties Of Metals On The Periodic Table Metals are located on the left side and the middle of the periodic table. The position of an element on the periodic table provides information about its properties. Most of the elements are metals. Group ia and group iia (the alkali metals) are the most active metals. The transition elements, groups ib. Metals include the alkali metal, alkaline earth, transition. What Are The Properties Of Metals On The Periodic Table.
From dashamlav.com
Periodic Table Groups Names and Properties What Are The Properties Of Metals On The Periodic Table Most of the elements are metals. Metals are solids at room temperature with the. Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. The transition elements, groups ib. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Group ia and. What Are The Properties Of Metals On The Periodic Table.
From www.chemistrylearner.com
Periodic Trends Definition and Properties What Are The Properties Of Metals On The Periodic Table Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metals are solids at room temperature with the. Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. Metals are solids at room temperature with the. Metals are located on the left side and the middle. What Are The Properties Of Metals On The Periodic Table.
From schoolbag.info
Figure 2.3. Periodic Table, Coded by Element Type What Are The Properties Of Metals On The Periodic Table Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. The transition elements, groups ib. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metals are located on the left side and the middle of the periodic table. The majority of elements are metals and. What Are The Properties Of Metals On The Periodic Table.
From utedzz.blogspot.com
Periodic Table Of Elements With Metals Nonmetals Metalloids And Noble What Are The Properties Of Metals On The Periodic Table The position of an element on the periodic table provides information about its properties. Metals are located on the left side and the middle of the periodic table. Most of the elements are metals. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. The majority of elements are metals and they are found on the left and in. What Are The Properties Of Metals On The Periodic Table.
From periodictable.me
Labeled Periodic Table of Elements with Name What Are The Properties Of Metals On The Periodic Table Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Most of the elements are metals. Metals are solids at room temperature with the. Group ia and group iia (the alkali metals) are the most active metals. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. The transition elements, groups ib. Metals are located on the left. What Are The Properties Of Metals On The Periodic Table.
From www.slideserve.com
PPT The Periodic Table of Elements PowerPoint Presentation, free What Are The Properties Of Metals On The Periodic Table Metals are solids at room temperature with the. Group ia and group iia (the alkali metals) are the most active metals. Metals are solids at room temperature with the. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Metals are natural compounds of earth’s crust, in which they are generally found in the form. What Are The Properties Of Metals On The Periodic Table.
From studylib.net
printable periodic table of the elements What Are The Properties Of Metals On The Periodic Table Group ia and group iia (the alkali metals) are the most active metals. The position of an element on the periodic table provides information about its properties. The majority of elements are metals and they are found on the left and in the. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Metals are. What Are The Properties Of Metals On The Periodic Table.
From utedzz.blogspot.com
Periodic Table With Metals Metalloids And Nonmetals Labeled Periodic What Are The Properties Of Metals On The Periodic Table The transition elements, groups ib. Metals are solids at room temperature with the. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Most of the elements are metals. Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. Metals are solids at room temperature with. What Are The Properties Of Metals On The Periodic Table.
From commons.wikimedia.org
FilePeriodic Table Atomic Properties of the Elements.png What Are The Properties Of Metals On The Periodic Table Group ia and group iia (the alkali metals) are the most active metals. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metals are solids at room temperature with the. Most of the elements are metals. The position of an element on the periodic table provides information about its properties. Metals include the alkali metal, alkaline earth, transition. What Are The Properties Of Metals On The Periodic Table.
From ar.inspiredpencil.com
Periodic Table Of Elements With Everything Labeled On It What Are The Properties Of Metals On The Periodic Table The position of an element on the periodic table provides information about its properties. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. The transition elements, groups ib. Metals are solids at room temperature with the. Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with. What Are The Properties Of Metals On The Periodic Table.
From www.angelo.edu
The Parts of the Periodic Table What Are The Properties Of Metals On The Periodic Table Group ia and group iia (the alkali metals) are the most active metals. The position of an element on the periodic table provides information about its properties. Metals are located on the left side and the middle of the periodic table. Metals are solids at room temperature with the. Metals are lustrous, malleable, ductile, good conductors of heat and electricity.. What Are The Properties Of Metals On The Periodic Table.
From periodictableguide.com
Different Types of Metals on the Periodic table (With Image) What Are The Properties Of Metals On The Periodic Table Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. The majority of elements are metals and they are found on the left and in the. Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. The transition elements, groups ib. Group. What Are The Properties Of Metals On The Periodic Table.
From www.periodictableprintable.com
Description Of Metals On The Periodic Table Periodic Table Printable What Are The Properties Of Metals On The Periodic Table Most of the elements are metals. The position of an element on the periodic table provides information about its properties. The majority of elements are metals and they are found on the left and in the. Metals are solids at room temperature with the. Metals are natural compounds of earth’s crust, in which they are generally found in the form. What Are The Properties Of Metals On The Periodic Table.
From www.bbc.co.uk
What are metals and nonmetals on the periodic table? BBC Bitesize What Are The Properties Of Metals On The Periodic Table Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. The position of an element on the periodic table provides information about its properties. The transition elements, groups ib. Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. Metals are lustrous,. What Are The Properties Of Metals On The Periodic Table.
From www.thoughtco.com
Properties of Periodic Table of Element Groups What Are The Properties Of Metals On The Periodic Table Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. Metals are solids at room temperature with the. Metals are located on the left side and the middle of the periodic table. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Group ia and group. What Are The Properties Of Metals On The Periodic Table.
From examples.yourdictionary.com
Basic Types of Metals on the Periodic Table What Are The Properties Of Metals On The Periodic Table Metals are solids at room temperature with the. Most of the elements are metals. Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. The majority of elements are metals and they are found on the left and in the. Metals are solids at room temperature with. What Are The Properties Of Metals On The Periodic Table.
From www.slideserve.com
PPT Organization of The Periodic Table PowerPoint Presentation, free What Are The Properties Of Metals On The Periodic Table Metals are located on the left side and the middle of the periodic table. The majority of elements are metals and they are found on the left and in the. The position of an element on the periodic table provides information about its properties. Most of the elements are metals. Metals are natural compounds of earth’s crust, in which they. What Are The Properties Of Metals On The Periodic Table.
From www.britannica.com
transition metal Definition, Properties, Elements, & Facts Britannica What Are The Properties Of Metals On The Periodic Table Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metals are solids at room temperature with the. The transition elements, groups ib. The position of an element on the periodic table provides information about its. What Are The Properties Of Metals On The Periodic Table.
From courses.lumenlearning.com
Occurrence, Preparation, and Properties of Transition Metals and Their What Are The Properties Of Metals On The Periodic Table The transition elements, groups ib. Metals are solids at room temperature with the. Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metals are located on the left side and the middle of the periodic. What Are The Properties Of Metals On The Periodic Table.
From bucarotechelp.com
Brief Description of the Chemical and Physical Properties of Elements What Are The Properties Of Metals On The Periodic Table Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Most of the elements are metals. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. The transition elements, groups ib. Metals are solids at room temperature with the. Metals are located on the left side and the middle of the periodic table. Metals. What Are The Properties Of Metals On The Periodic Table.
From periodictableguide.com
Periodic table labeled with Metals Nonmetals and Metalloids What Are The Properties Of Metals On The Periodic Table The position of an element on the periodic table provides information about its properties. Metals are located on the left side and the middle of the periodic table. Group ia and group iia (the alkali metals) are the most active metals. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. The transition elements, groups. What Are The Properties Of Metals On The Periodic Table.
From www.thoughtco.com
The Periodic Properties of the Elements What Are The Properties Of Metals On The Periodic Table Metals are located on the left side and the middle of the periodic table. Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. The transition elements, groups ib. The position of an element on the. What Are The Properties Of Metals On The Periodic Table.
From chemistry.about.com
Practice Using the Periodic Table to Find Element Facts What Are The Properties Of Metals On The Periodic Table Metals are located on the left side and the middle of the periodic table. Most of the elements are metals. Metals are solids at room temperature with the. Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. Metals are solids at room temperature with the. Metals. What Are The Properties Of Metals On The Periodic Table.
From www.periodictableprintable.com
Elements & Periodic Table Periodic Table Printable What Are The Properties Of Metals On The Periodic Table Metals are located on the left side and the middle of the periodic table. Metals are solids at room temperature with the. The position of an element on the periodic table provides information about its properties. Group ia and group iia (the alkali metals) are the most active metals. Metals are lustrous, malleable, ductile, good conductors of heat and electricity.. What Are The Properties Of Metals On The Periodic Table.
From glossary.periodni.com
Periodic table Chemistry Dictionary & Glossary What Are The Properties Of Metals On The Periodic Table The transition elements, groups ib. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. The majority of elements are metals and they are found on the left and in the. Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. Metals are lustrous, malleable, ductile,. What Are The Properties Of Metals On The Periodic Table.
From sciencenotes.org
List of Metals What Are The Properties Of Metals On The Periodic Table The position of an element on the periodic table provides information about its properties. Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each. The transition elements, groups ib. The majority of elements are metals and they are found on the left and in the. Metals are. What Are The Properties Of Metals On The Periodic Table.
From interestingengineering.com
The complete list of metals on the periodic table and their reallife What Are The Properties Of Metals On The Periodic Table The transition elements, groups ib. Most of the elements are metals. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metals are solids at room temperature with the. The position of an element on the periodic table provides information about its properties. Group ia and group iia (the alkali metals) are the most active metals. Metals are lustrous,. What Are The Properties Of Metals On The Periodic Table.
From sciencenotes.org
How To Use a Periodic Table What Are The Properties Of Metals On The Periodic Table Most of the elements are metals. The transition elements, groups ib. The majority of elements are metals and they are found on the left and in the. Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Metals are solids at room temperature with the. The position of an element on the periodic table provides information about its properties.. What Are The Properties Of Metals On The Periodic Table.
From scienceaplus.com
All Metals on the periodic table Science A Plus What Are The Properties Of Metals On The Periodic Table The transition elements, groups ib. Metals are solids at room temperature with the. Group ia and group iia (the alkali metals) are the most active metals. The position of an element on the periodic table provides information about its properties. Metals are located on the left side and the middle of the periodic table. Metals are lustrous, malleable, ductile, good. What Are The Properties Of Metals On The Periodic Table.