Hydrogen Electrode In Solution . Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. Theoretically, it is the most important electrode. The outer hydrogen electrode measures the hydrogen potential in the measuring solution. The platinum electrode consists of a tiny platinum foil square that is platinized. Against a normal hydrogen electrode (per definition: The structure of the standard hydrogen electrode is. The hydrogen electrode is used as a reference for electrode potential measurements. The value of the standard electrode potential is zero, which forms. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution.
from askfilo.com
The structure of the standard hydrogen electrode is. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. The platinum electrode consists of a tiny platinum foil square that is platinized. The hydrogen electrode is used as a reference for electrode potential measurements. Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. Against a normal hydrogen electrode (per definition: The outer hydrogen electrode measures the hydrogen potential in the measuring solution. Theoretically, it is the most important electrode. The value of the standard electrode potential is zero, which forms.
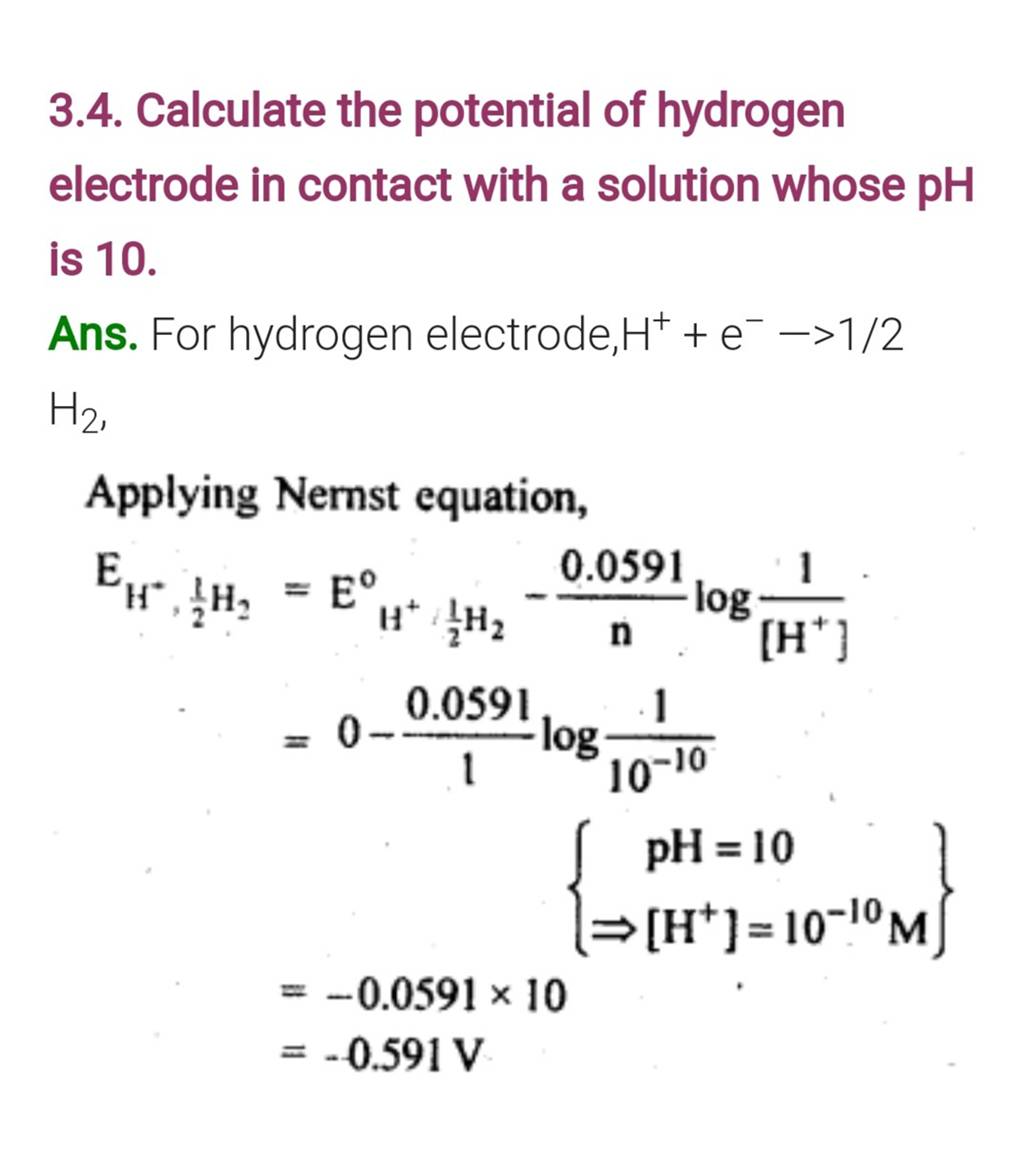
3.4. Calculate the potential of hydrogen electrode in contact with a solu..
Hydrogen Electrode In Solution Theoretically, it is the most important electrode. Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. Against a normal hydrogen electrode (per definition: The platinum electrode consists of a tiny platinum foil square that is platinized. The value of the standard electrode potential is zero, which forms. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. The hydrogen electrode is used as a reference for electrode potential measurements. Theoretically, it is the most important electrode. The outer hydrogen electrode measures the hydrogen potential in the measuring solution. The structure of the standard hydrogen electrode is.
From solvedlib.com
3 Draw a labeled diagram of standard hydrogen electro… SolvedLib Hydrogen Electrode In Solution The hydrogen electrode is used as a reference for electrode potential measurements. Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. Against a normal hydrogen electrode (per definition: The outer hydrogen electrode measures the hydrogen potential in the measuring. Hydrogen Electrode In Solution.
From www.youtube.com
A hydrogen electrode is dipped in a solution at `25^()C`. The Hydrogen Electrode In Solution Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. Against a normal hydrogen electrode (per definition: The outer hydrogen electrode measures the hydrogen potential in the measuring solution. The platinum electrode consists of a tiny platinum foil square that is platinized. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in. Hydrogen Electrode In Solution.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Hydrogen Electrode In Solution The structure of the standard hydrogen electrode is. The hydrogen electrode is used as a reference for electrode potential measurements. The value of the standard electrode potential is zero, which forms. The platinum electrode consists of a tiny platinum foil square that is platinized. The outer hydrogen electrode measures the hydrogen potential in the measuring solution. Against a normal hydrogen. Hydrogen Electrode In Solution.
From www.savemyexams.com
The Hydrogen Electrode (HL) HL IB Chemistry Revision Notes 2025 Hydrogen Electrode In Solution The outer hydrogen electrode measures the hydrogen potential in the measuring solution. Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. The hydrogen electrode is used as a reference for electrode potential measurements. The structure of the standard hydrogen electrode is. The platinum electrode consists of a tiny platinum foil square that is platinized. Against a. Hydrogen Electrode In Solution.
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that Hydrogen Electrode In Solution The outer hydrogen electrode measures the hydrogen potential in the measuring solution. The value of the standard electrode potential is zero, which forms. The platinum electrode consists of a tiny platinum foil square that is platinized. Against a normal hydrogen electrode (per definition: Theoretically, it is the most important electrode. Hydrogen gas bubbles around the platinum electrode at a pressure. Hydrogen Electrode In Solution.
From gaskatel.de
Usage of the hydrogen electrodes HydroFlex and MiniHydroFlex Gaskatel Hydrogen Electrode In Solution Against a normal hydrogen electrode (per definition: Theoretically, it is the most important electrode. The structure of the standard hydrogen electrode is. The hydrogen electrode is used as a reference for electrode potential measurements. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. The platinum electrode consists of a tiny platinum foil. Hydrogen Electrode In Solution.
From en.ppt-online.org
Electrochemistry. Oxidationreduction equilibrium in water solutions Hydrogen Electrode In Solution Theoretically, it is the most important electrode. Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. The platinum electrode consists of a tiny platinum foil square that is platinized. The outer hydrogen electrode measures the hydrogen potential in the measuring solution. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in. Hydrogen Electrode In Solution.
From mavink.com
Hydrogen Electrode Diagram Hydrogen Electrode In Solution The outer hydrogen electrode measures the hydrogen potential in the measuring solution. The value of the standard electrode potential is zero, which forms. The structure of the standard hydrogen electrode is. Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a. Hydrogen Electrode In Solution.
From www.toppr.com
Calculate the potential of hydrogen electrode in contact with a Hydrogen Electrode In Solution Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. Theoretically, it is the most important electrode. The hydrogen electrode is used as a reference for electrode potential measurements. Against a normal hydrogen electrode (per definition: The outer hydrogen electrode measures the hydrogen potential in the measuring solution. The value of the standard electrode potential is zero,. Hydrogen Electrode In Solution.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Hydrogen Electrode In Solution She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. The structure of the standard hydrogen electrode is. Theoretically, it is the most important electrode. Against a normal hydrogen electrode (per definition: Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. The value of the standard electrode potential is. Hydrogen Electrode In Solution.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Hydrogen Electrode In Solution Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. The hydrogen electrode is used as a reference for electrode potential measurements. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. The value of the standard electrode potential is zero, which forms. The platinum electrode consists of a tiny. Hydrogen Electrode In Solution.
From byjus.com
A hydrogen electrode placed in a buffer solution of CH3COOH and acetic Hydrogen Electrode In Solution Against a normal hydrogen electrode (per definition: The hydrogen electrode is used as a reference for electrode potential measurements. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. The structure of the standard hydrogen electrode is. The value of the standard electrode potential is zero, which forms. The platinum electrode consists of. Hydrogen Electrode In Solution.
From www.youtube.com
Standard Hydrogen Electrode (SHE)/ Primary reference electrode Gas Hydrogen Electrode In Solution She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. The platinum electrode consists of a tiny platinum foil square that is platinized. Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. The structure of the standard hydrogen electrode is. Against a normal hydrogen electrode (per definition: The value. Hydrogen Electrode In Solution.
From www.numerade.com
The electrode potential of hydrogen electrode in neutral solution and Hydrogen Electrode In Solution The outer hydrogen electrode measures the hydrogen potential in the measuring solution. The hydrogen electrode is used as a reference for electrode potential measurements. Theoretically, it is the most important electrode. The structure of the standard hydrogen electrode is. The platinum electrode consists of a tiny platinum foil square that is platinized. She consists of a platinum electrode with a. Hydrogen Electrode In Solution.
From www.slideserve.com
PPT Chapter 21 Electrochemistry PowerPoint Presentation, free Hydrogen Electrode In Solution The structure of the standard hydrogen electrode is. The hydrogen electrode is used as a reference for electrode potential measurements. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. Against a normal hydrogen electrode (per definition: Theoretically, it is the most important electrode. The value of the standard electrode potential is zero,. Hydrogen Electrode In Solution.
From stock.adobe.com
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use Hydrogen Electrode In Solution Theoretically, it is the most important electrode. The hydrogen electrode is used as a reference for electrode potential measurements. The value of the standard electrode potential is zero, which forms. Against a normal hydrogen electrode (per definition: Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. The structure of the standard hydrogen electrode is. She consists. Hydrogen Electrode In Solution.
From www.youtube.com
Calculate the potential of hydrogen electrode in contact with a Hydrogen Electrode In Solution Theoretically, it is the most important electrode. The hydrogen electrode is used as a reference for electrode potential measurements. The platinum electrode consists of a tiny platinum foil square that is platinized. The outer hydrogen electrode measures the hydrogen potential in the measuring solution. The value of the standard electrode potential is zero, which forms. Hydrogen gas bubbles around the. Hydrogen Electrode In Solution.
From chemistnotes.com
Standard hydrogen electrode(SHE) Definition, diagram, application Hydrogen Electrode In Solution She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. The structure of the standard hydrogen electrode is. Against a normal hydrogen electrode (per definition: The platinum electrode consists of a tiny platinum foil square that is platinized. The value. Hydrogen Electrode In Solution.
From monomole.com
Standard hydrogen electrode Mono Mole Hydrogen Electrode In Solution The platinum electrode consists of a tiny platinum foil square that is platinized. The value of the standard electrode potential is zero, which forms. Against a normal hydrogen electrode (per definition: The structure of the standard hydrogen electrode is. The outer hydrogen electrode measures the hydrogen potential in the measuring solution. Theoretically, it is the most important electrode. She consists. Hydrogen Electrode In Solution.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Hydrogen Electrode In Solution She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. Theoretically, it is the most important electrode. The outer hydrogen electrode measures the hydrogen potential in the measuring solution. The hydrogen electrode is used as a reference for electrode potential measurements. The value of the standard electrode potential is zero, which forms. Hydrogen. Hydrogen Electrode In Solution.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Hydrogen Electrode In Solution The value of the standard electrode potential is zero, which forms. Against a normal hydrogen electrode (per definition: The structure of the standard hydrogen electrode is. Theoretically, it is the most important electrode. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. The hydrogen electrode is used as a reference for electrode. Hydrogen Electrode In Solution.
From saylordotorg.github.io
Electrochemistry Hydrogen Electrode In Solution The value of the standard electrode potential is zero, which forms. The hydrogen electrode is used as a reference for electrode potential measurements. Against a normal hydrogen electrode (per definition: The structure of the standard hydrogen electrode is. Theoretically, it is the most important electrode. Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. The outer. Hydrogen Electrode In Solution.
From www.youtube.com
Standard Hydrogen Electrode Demonstration YouTube Hydrogen Electrode In Solution Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. The value of the standard electrode potential is zero, which forms. The platinum electrode consists of a tiny platinum foil square that is platinized. The outer hydrogen electrode measures the. Hydrogen Electrode In Solution.
From twitter.com
Digital Kemistry Free Online Chemistry Learning on Twitter "Do you Hydrogen Electrode In Solution The structure of the standard hydrogen electrode is. The outer hydrogen electrode measures the hydrogen potential in the measuring solution. Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. Against a normal hydrogen electrode (per definition: The value of the standard electrode potential is zero, which forms. Theoretically, it is the most important electrode. She consists. Hydrogen Electrode In Solution.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Hydrogen Electrode In Solution Theoretically, it is the most important electrode. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. The value of the standard electrode potential is zero, which forms. The platinum electrode consists of a tiny platinum foil square that is. Hydrogen Electrode In Solution.
From study.com
Electrolysis of Aqueous Solutions Lesson Hydrogen Electrode In Solution Theoretically, it is the most important electrode. The outer hydrogen electrode measures the hydrogen potential in the measuring solution. The value of the standard electrode potential is zero, which forms. The structure of the standard hydrogen electrode is. The platinum electrode consists of a tiny platinum foil square that is platinized. Hydrogen gas bubbles around the platinum electrode at a. Hydrogen Electrode In Solution.
From byjus.com
What is standard hydrogen electrode? Hydrogen Electrode In Solution Theoretically, it is the most important electrode. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. The hydrogen electrode is used as a reference for electrode potential measurements. Against a normal hydrogen electrode (per definition: The platinum electrode consists of a tiny platinum foil square that is platinized. The outer hydrogen electrode. Hydrogen Electrode In Solution.
From question.pandai.org
Standard Electrode Potential Hydrogen Electrode In Solution The structure of the standard hydrogen electrode is. Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. Against a normal hydrogen electrode (per definition: Theoretically, it is the most important electrode. The outer hydrogen electrode measures the hydrogen potential in the measuring solution. The hydrogen electrode is used as a reference for electrode potential measurements. The. Hydrogen Electrode In Solution.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Hydrogen Electrode In Solution The structure of the standard hydrogen electrode is. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. The hydrogen electrode is used as a reference for electrode potential measurements. The outer hydrogen electrode measures the hydrogen potential in the measuring solution. The platinum electrode consists of a tiny platinum foil square that. Hydrogen Electrode In Solution.
From askfilo.com
3.4. Calculate the potential of hydrogen electrode in contact with a solu.. Hydrogen Electrode In Solution Against a normal hydrogen electrode (per definition: The platinum electrode consists of a tiny platinum foil square that is platinized. Theoretically, it is the most important electrode. The outer hydrogen electrode measures the hydrogen potential in the measuring solution. Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere. The hydrogen electrode is used as a reference. Hydrogen Electrode In Solution.
From scienceinfo.com
What is Standard Hydrogen Electrode? Hydrogen Electrode In Solution The value of the standard electrode potential is zero, which forms. Against a normal hydrogen electrode (per definition: Theoretically, it is the most important electrode. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. The platinum electrode consists of a tiny platinum foil square that is platinized. The outer hydrogen electrode measures. Hydrogen Electrode In Solution.
From www.youtube.com
Standard Hydrogen Electrode Definition ,Construction ,Working Hydrogen Electrode In Solution The value of the standard electrode potential is zero, which forms. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m submerged in a solution. The hydrogen electrode is used as a reference for electrode potential measurements. Against a normal hydrogen electrode (per definition: The outer hydrogen electrode measures the hydrogen potential in the measuring solution. Theoretically,. Hydrogen Electrode In Solution.
From www.shutterstock.com
Diagramme standard d'électrode à hydrogène. Illustration image Hydrogen Electrode In Solution The value of the standard electrode potential is zero, which forms. The platinum electrode consists of a tiny platinum foil square that is platinized. Theoretically, it is the most important electrode. The hydrogen electrode is used as a reference for electrode potential measurements. The outer hydrogen electrode measures the hydrogen potential in the measuring solution. Against a normal hydrogen electrode. Hydrogen Electrode In Solution.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID752723 Hydrogen Electrode In Solution The outer hydrogen electrode measures the hydrogen potential in the measuring solution. Theoretically, it is the most important electrode. The structure of the standard hydrogen electrode is. The platinum electrode consists of a tiny platinum foil square that is platinized. The value of the standard electrode potential is zero, which forms. Hydrogen gas bubbles around the platinum electrode at a. Hydrogen Electrode In Solution.
From saylordotorg.github.io
Electrochemistry Hydrogen Electrode In Solution The platinum electrode consists of a tiny platinum foil square that is platinized. Against a normal hydrogen electrode (per definition: The structure of the standard hydrogen electrode is. Theoretically, it is the most important electrode. The hydrogen electrode is used as a reference for electrode potential measurements. She consists of a platinum electrode with a hydrogen ion concentration of 1.00m. Hydrogen Electrode In Solution.