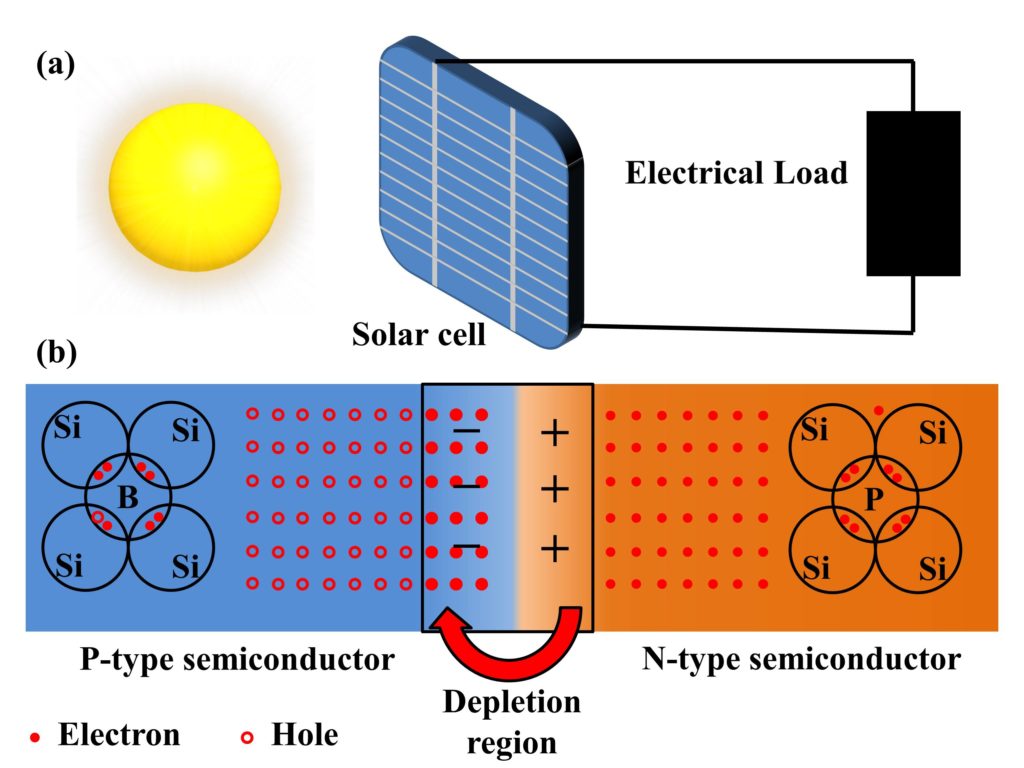
What Is The Principle Of Electric Cell . These electrodes are immersed in the electrolytic. An electrical cell is an “electrical power supply” that converts chemical energy. Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. Reduction describes the gain of electrons by a molecule, atom, or ion. Cells provide electric energy through chemical reactions. What is an electric cell: The electrons always flow from the. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a device that is used to maintain the electric. Oxidation describes the loss of electrons by a molecule, atom, or ion. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to.
from www.asrmeta.com
An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. These electrodes are immersed in the electrolytic. The electrons always flow from the. Reduction describes the gain of electrons by a molecule, atom, or ion. An electrical cell is an “electrical power supply” that converts chemical energy. What is an electric cell: An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a device that is used to maintain the electric. Oxidation describes the loss of electrons by a molecule, atom, or ion.
Solar cells, their construction, and working
What Is The Principle Of Electric Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Cells provide electric energy through chemical reactions. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a device that is used to maintain the electric. Reduction describes the gain of electrons by a molecule, atom, or ion. These electrodes are immersed in the electrolytic. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. The electrons always flow from the. What is an electric cell: Oxidation describes the loss of electrons by a molecule, atom, or ion. Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. An electrical cell is an “electrical power supply” that converts chemical energy.
From www.electricity-magnetism.org
Battery Principle of operation Electricity What Is The Principle Of Electric Cell An electrical cell is an “electrical power supply” that converts chemical energy. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Reduction describes the gain of electrons by a molecule, atom, or ion. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons. What Is The Principle Of Electric Cell.
From www.yourelectricalguide.com
Fuel Cell Working Principle your electrical guide What Is The Principle Of Electric Cell The electrons always flow from the. Reduction describes the gain of electrons by a molecule, atom, or ion. What is an electric cell: An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous.. What Is The Principle Of Electric Cell.
From www.findlight.net
Solar Cell History and Milestones What Is The Principle Of Electric Cell An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a device that is used to maintain the electric. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. These electrodes are immersed in the electrolytic. What is an electric cell: An apparatus that is used to. What Is The Principle Of Electric Cell.
From studiousguy.com
Fuel Cell Working Principle StudiousGuy What Is The Principle Of Electric Cell Reduction describes the gain of electrons by a molecule, atom, or ion. An electrical cell is an “electrical power supply” that converts chemical energy. Oxidation describes the loss of electrons by a molecule, atom, or ion. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Cells provide electric energy through chemical reactions. These electrodes. What Is The Principle Of Electric Cell.
From www.visualcapitalist.com
Animated Infographic How Solar Panels Work What Is The Principle Of Electric Cell Oxidation describes the loss of electrons by a molecule, atom, or ion. The electrons always flow from the. What is an electric cell: These electrodes are immersed in the electrolytic. Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. An electrical cell is an “electrical power supply” that converts chemical. What Is The Principle Of Electric Cell.
From www.chfca.ca
About Fuel Cells CHFCA What Is The Principle Of Electric Cell The electrons always flow from the. An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a device that is used to maintain the electric. An electrical cell is an “electrical power supply” that converts chemical energy. Oxidation describes the loss of electrons by a molecule, atom, or ion. Reduction describes the. What Is The Principle Of Electric Cell.
From www.electronicsandyou.com
PV Cell Working Principle How Solar Photovoltaic Cells Work What Is The Principle Of Electric Cell An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a device that is used to maintain the electric. Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. The electrons always flow from the. Oxidation describes the loss of electrons by a. What Is The Principle Of Electric Cell.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle What Is The Principle Of Electric Cell Cells provide electric energy through chemical reactions. Reduction describes the gain of electrons by a molecule, atom, or ion. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. What is an electric cell: An electrochemical cell splits the oxidant and reductant in a manner that allows electrons. What Is The Principle Of Electric Cell.
From www.alamy.com
Fuel cell diagram. Vector. Device that converts chemical potential What Is The Principle Of Electric Cell These electrodes are immersed in the electrolytic. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrical cell is an “electrical power supply” that converts chemical energy. Oxidation describes the loss of electrons by a molecule, atom, or ion. The electrons always flow from the. Electric cells are devices that convert chemical energy. What Is The Principle Of Electric Cell.
From electricala2z.com
Figure1Functionaldiagramoffuelcell Electrical A2Z What Is The Principle Of Electric Cell An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a device that is used to maintain the electric. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. The electrons always flow from the. Reduction describes the gain of electrons by a molecule, atom, or ion.. What Is The Principle Of Electric Cell.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science What Is The Principle Of Electric Cell An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a device that is used to maintain the electric. An electrical cell is an “electrical power supply” that converts chemical energy. These electrodes are immersed in the electrolytic. Oxidation describes the loss of electrons by a molecule, atom, or ion. Electric cells. What Is The Principle Of Electric Cell.
From www.youtube.com
Electric cell diagram drawing/How to draw Electric cell labeled diagram What Is The Principle Of Electric Cell Oxidation describes the loss of electrons by a molecule, atom, or ion. The electrons always flow from the. Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. What is an electric cell: Cells provide electric energy through chemical reactions. An electric cell is an electrical power supply that generates electrical. What Is The Principle Of Electric Cell.
From www.mech4study.com
How a Solar Power Plant Works and What are main Types of it? mech4study What Is The Principle Of Electric Cell The electrons always flow from the. What is an electric cell: Oxidation describes the loss of electrons by a molecule, atom, or ion. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Reduction describes the gain of electrons by a molecule, atom, or ion. An electric cell. What Is The Principle Of Electric Cell.
From electricalnotebook.com
Plot IV Characteristics of Photovoltaic Cell Module and Find Out the What Is The Principle Of Electric Cell These electrodes are immersed in the electrolytic. An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a device that is used to maintain the electric. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Electric cells are devices that convert chemical energy into electrical energy. What Is The Principle Of Electric Cell.
From webmotor.org
What Is The Principle Of Motor What Is The Principle Of Electric Cell Reduction describes the gain of electrons by a molecule, atom, or ion. These electrodes are immersed in the electrolytic. What is an electric cell: Cells provide electric energy through chemical reactions. The electrons always flow from the. Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. An electrochemical cell splits. What Is The Principle Of Electric Cell.
From www.electricaltechnology.org
How To Make Simple Solar Cell? Working of Photovoltaic Cell What Is The Principle Of Electric Cell Reduction describes the gain of electrons by a molecule, atom, or ion. Oxidation describes the loss of electrons by a molecule, atom, or ion. The electrons always flow from the. Cells provide electric energy through chemical reactions. Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. An electrical cell is. What Is The Principle Of Electric Cell.
From sinovoltaics.com
Photoelectrochemical Cell What Is The Principle Of Electric Cell An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a device that is used to maintain the electric. These electrodes are immersed in the electrolytic. Cells provide electric energy through chemical reactions. What is an electric cell: An apparatus that is used to generate electricity from a spontaneous redox reaction or,. What Is The Principle Of Electric Cell.
From www.treatumedical.com
The of Cell Physiology 慈佑醫學科技 What Is The Principle Of Electric Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Oxidation describes the loss of electrons by a molecule, atom, or ion. Cells provide electric energy through chemical reactions. Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. An apparatus that is used to generate. What Is The Principle Of Electric Cell.
From cosmosmagazine.com
How solar cells turn sunlight into electricity Cosmos Magazine What Is The Principle Of Electric Cell What is an electric cell: These electrodes are immersed in the electrolytic. The electrons always flow from the. Oxidation describes the loss of electrons by a molecule, atom, or ion. Reduction describes the gain of electrons by a molecule, atom, or ion. An electrical cell is an “electrical power supply” that converts chemical energy. An electric cell is an electrical. What Is The Principle Of Electric Cell.
From brainly.in
draw and describe the parts of an electric cell Brainly.in What Is The Principle Of Electric Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Oxidation describes the loss of electrons by a molecule, atom, or ion. What is an electric cell: Cells provide electric energy through chemical. What Is The Principle Of Electric Cell.
From www.topperlearning.com
how does a dry cell work ijitmtuu What Is The Principle Of Electric Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. What is an electric cell: An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a device that is used to maintain the electric. These electrodes are immersed in the. What Is The Principle Of Electric Cell.
From www.asrmeta.com
Solar cells, their construction, and working What Is The Principle Of Electric Cell What is an electric cell: Cells provide electric energy through chemical reactions. The electrons always flow from the. These electrodes are immersed in the electrolytic. An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a device that is used to maintain the electric. Oxidation describes the loss of electrons by a. What Is The Principle Of Electric Cell.
From www.vecteezy.com
Circuit Diagram Vector Art, Icons, and Graphics for Free Download What Is The Principle Of Electric Cell The electrons always flow from the. Oxidation describes the loss of electrons by a molecule, atom, or ion. Reduction describes the gain of electrons by a molecule, atom, or ion. What is an electric cell: Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. Cells provide electric energy through chemical. What Is The Principle Of Electric Cell.
From www.newscientist.com
Building the battery of the future today New Scientist What Is The Principle Of Electric Cell An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a device that is used to maintain the electric. Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. An electrical cell is an “electrical power supply” that converts chemical energy. Reduction describes. What Is The Principle Of Electric Cell.
From www.youtube.com
Electric Cell All you need to know!! Class 6 Electricity and What Is The Principle Of Electric Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. The electrons always flow from the. An electrical cell is an “electrical power supply” that converts chemical energy. These electrodes are immersed in the electrolytic. Oxidation describes the loss of electrons by a molecule, atom, or ion. Reduction. What Is The Principle Of Electric Cell.
From classnotes.org.in
Batteries Chemistry, Class 12, Electro Chemistry What Is The Principle Of Electric Cell Cells provide electric energy through chemical reactions. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. Reduction describes the gain of electrons by a molecule, atom, or ion.. What Is The Principle Of Electric Cell.
From www.britannica.com
Alkaline cell battery Britannica What Is The Principle Of Electric Cell Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. Cells provide electric energy through chemical reactions. What is an electric cell: The electrons always flow from the. These electrodes are immersed in the electrolytic. Oxidation describes the loss of electrons by a molecule, atom, or ion. An apparatus that is. What Is The Principle Of Electric Cell.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki What Is The Principle Of Electric Cell Oxidation describes the loss of electrons by a molecule, atom, or ion. These electrodes are immersed in the electrolytic. Reduction describes the gain of electrons by a molecule, atom, or ion. Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. The electrons always flow from the. An apparatus that is. What Is The Principle Of Electric Cell.
From www.brijencapsulants.com
Six Main Components of a Solar Panel Brij Encapsulants (India) What Is The Principle Of Electric Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrical cell is an “electrical power supply” that converts chemical energy. Oxidation describes the loss of electrons by a molecule, atom, or ion. Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. The electrons. What Is The Principle Of Electric Cell.
From www.atlearner.com
What is an Electric Cell? Definition, Types of Cell What Is The Principle Of Electric Cell An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a device that is used to maintain the electric. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Reduction describes the gain of electrons by a molecule, atom, or. What Is The Principle Of Electric Cell.
From www.slideserve.com
PPT Cells and Voltage PowerPoint Presentation, free download ID5231819 What Is The Principle Of Electric Cell These electrodes are immersed in the electrolytic. What is an electric cell: Cells provide electric energy through chemical reactions. Oxidation describes the loss of electrons by a molecule, atom, or ion. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Reduction describes the gain of electrons by. What Is The Principle Of Electric Cell.
From studiousguy.com
Solar Cell Working Principle StudiousGuy What Is The Principle Of Electric Cell Reduction describes the gain of electrons by a molecule, atom, or ion. The electrons always flow from the. These electrodes are immersed in the electrolytic. What is an electric cell: Oxidation describes the loss of electrons by a molecule, atom, or ion. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Cells provide electric. What Is The Principle Of Electric Cell.
From www.electroniclinic.com
Photovoltaic Effect or Solar Cell Construction and working What Is The Principle Of Electric Cell Electric cells are devices that convert chemical energy into electrical energy through a process involving oxidation (electron loss) and. An electrical cell is an “electrical power supply” that converts chemical energy. Reduction describes the gain of electrons by a molecule, atom, or ion. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. What Is The Principle Of Electric Cell.
From www.britannica.com
Primary cell electronics Britannica What Is The Principle Of Electric Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Cells provide electric energy through chemical reactions. These electrodes are immersed in the electrolytic. An electrical cell is an “electrical power supply” that converts chemical energy. An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a. What Is The Principle Of Electric Cell.
From www.nsta.org
The Photoelectric Effect NSTA What Is The Principle Of Electric Cell An electrical cell is an “electrical power supply” that converts chemical energy. Oxidation describes the loss of electrons by a molecule, atom, or ion. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons. What Is The Principle Of Electric Cell.