End Point Of Iodine Titration . The end point of the titration can therefore be difficult to see. Iodometry is one of the most important redox titration methods. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. In simple terms, titration is an analytical. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Repeat the titration using a second sample. The end point is the point when the violet iodine colour just disappears. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. If we add 2cm³ of starch solution, the reaction mixture will turn dark blue to. Titration with iodine or thiosulfate.
from slidetodoc.com
It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. The end point of the titration can therefore be difficult to see. Titration with iodine or thiosulfate. Iodometry is one of the most important redox titration methods. If we add 2cm³ of starch solution, the reaction mixture will turn dark blue to. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. The end point is the point when the violet iodine colour just disappears. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. In simple terms, titration is an analytical.
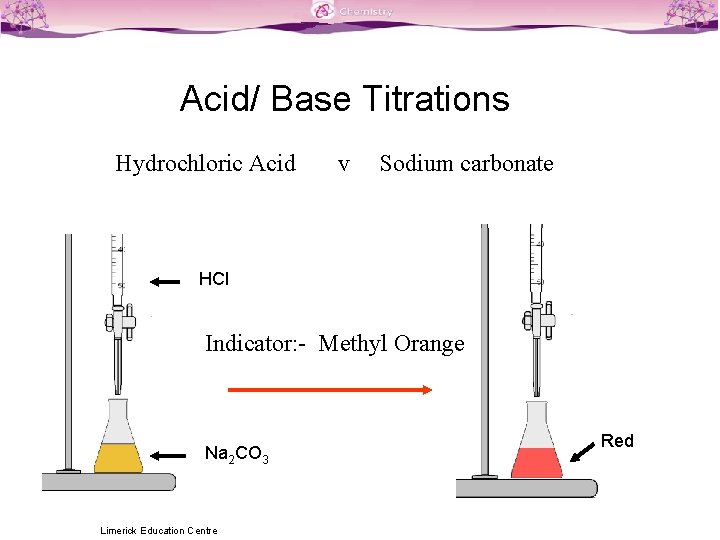
Titration Colour Changes SLSS Science Limerick Education Centre
End Point Of Iodine Titration Titration with iodine or thiosulfate. Repeat the titration using a second sample. Iodometry is one of the most important redox titration methods. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. In simple terms, titration is an analytical. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. The end point is the point when the violet iodine colour just disappears. If we add 2cm³ of starch solution, the reaction mixture will turn dark blue to. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. The end point of the titration can therefore be difficult to see. Titration with iodine or thiosulfate.
From www.alamy.com
Finding the end point of an iodine titration. 5 of 5. The titrant, a End Point Of Iodine Titration Repeat the titration using a second sample. Iodometry is one of the most important redox titration methods. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. Luckily high concentrations of iodine are easily. End Point Of Iodine Titration.
From www.numerade.com
SOLVED 0.6712 g of iodine sample was determined by the Volhard method End Point Of Iodine Titration Iodometry is one of the most important redox titration methods. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Titration with iodine or thiosulfate. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. The end point of the titration can therefore be. End Point Of Iodine Titration.
From pubs.rsc.org
Methods for detecting the endpoint in the titration of iodine with End Point Of Iodine Titration The end point of the titration can therefore be difficult to see. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. It works by mixing the oxidizing agent with. End Point Of Iodine Titration.
From www.alamy.com
Finding the end point of an iodine titration. 3 of 5. The titrant, a End Point Of Iodine Titration Iodometry is one of the most important redox titration methods. The end point of the titration can therefore be difficult to see. The end point is the point when the violet iodine colour just disappears. Repeat the titration using a second sample. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a. End Point Of Iodine Titration.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co End Point Of Iodine Titration Repeat the titration using a second sample. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. The equivalency. End Point Of Iodine Titration.
From www.numerade.com
SOLVED Vitamin C (ascorbic acid, C6H8O6, 176.12 g / mol ) can be End Point Of Iodine Titration The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. If we add 2cm³ of starch solution, the reaction mixture will turn dark blue to. Iodometry is one of the most important redox titration methods. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate. End Point Of Iodine Titration.
From www.sciencephoto.com
End point of an iodine titration. 2 of 5. Stock Image C029/1115 End Point Of Iodine Titration Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. The end point is the point when the violet iodine colour just disappears. If we add 2cm³ of starch solution, the reaction mixture will turn dark blue to. It works by mixing the oxidizing agent with iodide ions, which causes iodine. End Point Of Iodine Titration.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts End Point Of Iodine Titration Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Repeat the titration using a second sample. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. Iodometry is one of the most important redox titration methods. In simple terms, titration is. End Point Of Iodine Titration.
From saylordotorg.github.io
AcidBase Titrations End Point Of Iodine Titration Iodometry is one of the most important redox titration methods. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. Repeat the titration using a second sample. If we add 2cm³ of starch solution, the reaction mixture will turn dark blue to. The equivalency point, also known as the theoretical or. End Point Of Iodine Titration.
From www.youtube.com
Iodine and sodium thiosulfate redox titration calculations ALevel End Point Of Iodine Titration The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. The end point is the point when the violet iodine colour just disappears. In simple terms, titration is an analytical. Iodometry is one of the most important redox titration methods. Repeat the titration using a second sample. The end. End Point Of Iodine Titration.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free End Point Of Iodine Titration If we add 2cm³ of starch solution, the reaction mixture will turn dark blue to. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Repeat the titration using a second sample. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Iodometry is one of the most. End Point Of Iodine Titration.
From theedge.com.hk
Chemistry How To Titration The Edge End Point Of Iodine Titration In simple terms, titration is an analytical. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. If we add 2cm³ of starch solution, the reaction mixture will turn dark blue to. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. The equivalency. End Point Of Iodine Titration.
From www.vernier.com
Potentiometric Titration of Aqueous Iodine End Point Of Iodine Titration If we add 2cm³ of starch solution, the reaction mixture will turn dark blue to. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. Titration with iodine or thiosulfate. Repeat the titration using a second sample. In simple terms, titration is an analytical. The end point of the titration can. End Point Of Iodine Titration.
From naajennifermackenzie.blogspot.com
determination of iodine value experiment Jennifer Mackenzie End Point Of Iodine Titration Iodometry is one of the most important redox titration methods. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. In simple terms, titration is an analytical. Repeat the titration using a second sample.. End Point Of Iodine Titration.
From fineartamerica.com
End Point Of An Iodine Titration. 3 Of 5. Photograph by Martyn F End Point Of Iodine Titration Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. Repeat the titration using a second sample. Titration with iodine or thiosulfate. The end point of the titration can therefore. End Point Of Iodine Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre End Point Of Iodine Titration The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. In simple terms, titration is an analytical. Repeat the titration using a second sample. Titration with iodine or thiosulfate. The end point of the titration can therefore be difficult to see. Luckily high concentrations of iodine are easily visible,. End Point Of Iodine Titration.
From www.sliderbase.com
Titration End Point Of Iodine Titration It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Repeat the titration using a second sample. Iodometry is one of the most important redox titration methods. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. In simple terms, titration is an analytical.. End Point Of Iodine Titration.
From www.mdpi.com
Applied Sciences Free FullText Determination of Vitamin C in Foods End Point Of Iodine Titration In simple terms, titration is an analytical. If we add 2cm³ of starch solution, the reaction mixture will turn dark blue to. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. The end point is the point when the violet iodine colour just disappears. Titration with iodine or. End Point Of Iodine Titration.
From slideplayer.com
Titration Colour Changes ppt download End Point Of Iodine Titration It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Titration with iodine or thiosulfate. The end point is the point when the violet iodine colour just disappears. If we add 2cm³ of starch solution, the reaction mixture will turn dark blue to. Iodometry is one of the most important redox titration methods. Repeat the. End Point Of Iodine Titration.
From www.sciencephoto.com
End point of an iodine titration. 1 of 5. Stock Image C029/1076 End Point Of Iodine Titration If we add 2cm³ of starch solution, the reaction mixture will turn dark blue to. Repeat the titration using a second sample. The end point is the point when the violet iodine colour just disappears. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. Titration with iodine or thiosulfate. The. End Point Of Iodine Titration.
From www.vrogue.co
Ppt Exp 13 Volumetric Analysis Acid Base Titration Po vrogue.co End Point Of Iodine Titration Repeat the titration using a second sample. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to. End Point Of Iodine Titration.
From www.reddit.com
Titration using starch as an indicator for Iodine! At the end, one drop End Point Of Iodine Titration It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Iodometry is one of the most important redox titration methods. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. If we add 2cm³ of starch solution, the reaction mixture will turn dark blue to. Titration. End Point Of Iodine Titration.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS End Point Of Iodine Titration Titration with iodine or thiosulfate. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Iodometry is one of the most important redox titration methods. Repeat the titration using a second sample. The end point of the titration can therefore be difficult to see. Luckily high concentrations of iodine are easily visible, so. End Point Of Iodine Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre End Point Of Iodine Titration Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. If we add 2cm³ of starch solution, the reaction mixture will turn dark blue to. Luckily high concentrations of iodine are easily. End Point Of Iodine Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators End Point Of Iodine Titration Titration with iodine or thiosulfate. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. The end point is the point when the violet iodine colour just disappears. In simple. End Point Of Iodine Titration.
From fineartamerica.com
End Point Of An Iodine Titration. 5 Of 5. Photograph by Martyn F End Point Of Iodine Titration Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. In simple terms, titration is an analytical. The end point is the point when the violet iodine colour just disappears. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. Titration with. End Point Of Iodine Titration.
From www.youtube.com
Iodimetric titration standardization of thiosulfate YouTube End Point Of Iodine Titration In simple terms, titration is an analytical. Iodometry is one of the most important redox titration methods. Titration with iodine or thiosulfate. Repeat the titration using a second sample. The end point of the titration can therefore be difficult to see. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Luckily high concentrations of. End Point Of Iodine Titration.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation ID431911 End Point Of Iodine Titration Titration with iodine or thiosulfate. Iodometry is one of the most important redox titration methods. The end point of the titration can therefore be difficult to see. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. Luckily high concentrations of iodine are easily visible, so if we are. End Point Of Iodine Titration.
From www.alamy.com
Finding the end point of an iodine titration. 1 of 5. At the start of End Point Of Iodine Titration Titration with iodine or thiosulfate. Repeat the titration using a second sample. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. If we add 2cm³ of starch solution, the reaction mixture will turn dark blue. End Point Of Iodine Titration.
From childhealthpolicy.vumc.org
Vitamin c determination by iodine titration lab report. Vitamin C End Point Of Iodine Titration Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. The end point of the titration can therefore be difficult to see. The end point is the point when the violet iodine colour just. End Point Of Iodine Titration.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download End Point Of Iodine Titration The end point of the titration can therefore be difficult to see. The end point is the point when the violet iodine colour just disappears. In simple terms, titration is an analytical. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. It works by mixing the oxidizing agent. End Point Of Iodine Titration.
From www.alamy.com
Finding the end point of an iodine titration. At the start of an iodine End Point Of Iodine Titration Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. Iodometry is one of the most important redox titration methods. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. If we add 2cm³ of starch solution, the reaction mixture. End Point Of Iodine Titration.
From fineartamerica.com
End Point Of An Iodine Titration. 4 Of 5 Photograph by Martyn F End Point Of Iodine Titration Iodometry is one of the most important redox titration methods. Luckily high concentrations of iodine are easily visible, so if we are using thiosulfate to titrate solution that initially. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. The equivalency point, also known as the theoretical or stoichiometric end point, is the. End Point Of Iodine Titration.
From pixels.com
End Point Of An Iodine Titration. 1 Of 5. Photograph by Martyn F End Point Of Iodine Titration If we add 2cm³ of starch solution, the reaction mixture will turn dark blue to. The equivalency point, also known as the theoretical or stoichiometric end point, is the point at which a reaction is complete. Repeat the titration using a second sample. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be. Iodometry is. End Point Of Iodine Titration.
From www.sciencephoto.com
End point of an iodine titration. 5 of 5. Stock Image C029/1118 End Point Of Iodine Titration Titration with iodine or thiosulfate. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Repeat the titration using a second sample. The end point is the point when the violet iodine colour just disappears. In simple terms, titration is an analytical. Luckily high concentrations of iodine are easily visible, so if we. End Point Of Iodine Titration.