What Do You Mean By Standard Enthalpy Of Formation . The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free.
from www.youtube.com
A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from.
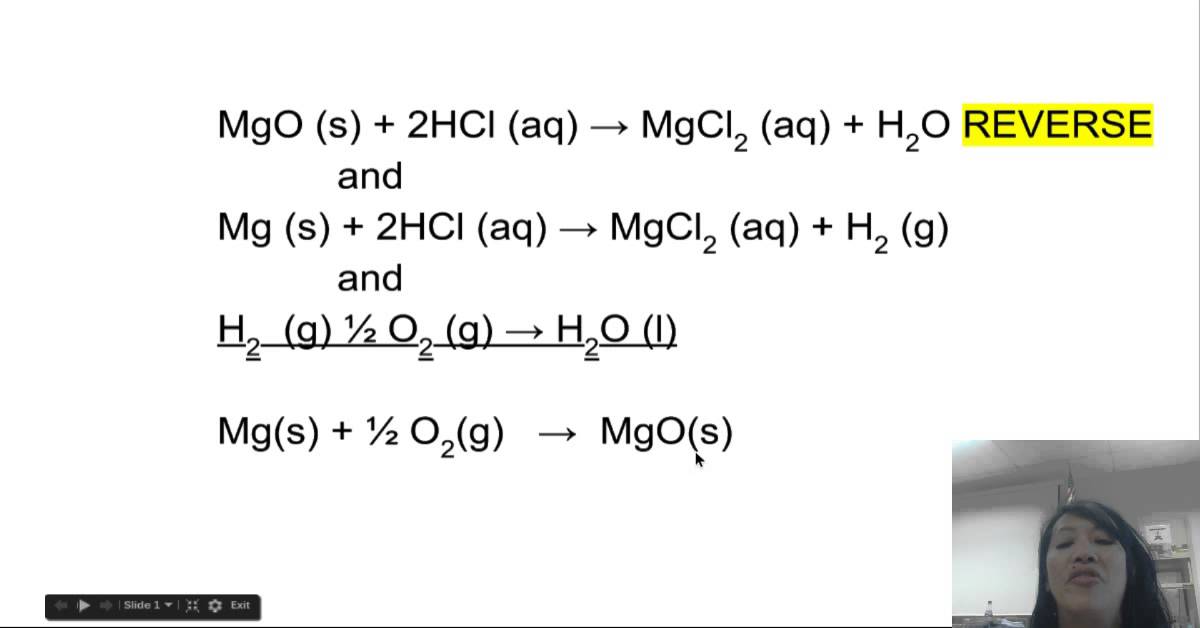
Heat of Formation of MgO YouTube
What Do You Mean By Standard Enthalpy Of Formation A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
From www.slideserve.com
PPT Higher Chemistry Unit 1(b) Enthalpy of combustion PowerPoint What Do You Mean By Standard Enthalpy Of Formation For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. What Do You Mean By Standard Enthalpy Of Formation.
From www.chegg.com
Solved Use The Standard Enthalpies Of Formation In The Ta... What Do You Mean By Standard Enthalpy Of Formation A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. Enthalpy of formation, also. What Do You Mean By Standard Enthalpy Of Formation.
From classmediawallopers.z14.web.core.windows.net
Heats Of Formation Formula What Do You Mean By Standard Enthalpy Of Formation For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed. What Do You Mean By Standard Enthalpy Of Formation.
From www.facebook.com
Super Cheat Powers and a Chill Adventure Awaits! Anime Full Episode 1 What Do You Mean By Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which. Enthalpy of formation,. What Do You Mean By Standard Enthalpy Of Formation.
From www.showme.com
Enthalpy of Reaction Science, Chemistry ShowMe What Do You Mean By Standard Enthalpy Of Formation Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. The standard enthalpy of formation. What Do You Mean By Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID What Do You Mean By Standard Enthalpy Of Formation Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. 193 rows. What Do You Mean By Standard Enthalpy Of Formation.
From www.vrogue.co
Hess S Law And Enthalpy Change Calculations vrogue.co What Do You Mean By Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy. What Do You Mean By Standard Enthalpy Of Formation.
From pressbooks.online.ucf.edu
10.4 Hess’s Law Chemistry Fundamentals What Do You Mean By Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for. What Do You Mean By Standard Enthalpy Of Formation.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture What Do You Mean By Standard Enthalpy Of Formation Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For any substance at any particular temperature, we define the standard enthalpy of formation as the. What Do You Mean By Standard Enthalpy Of Formation.
From learnah.org
2 Born Haber cycles What Do You Mean By Standard Enthalpy Of Formation The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. A standard enthalpy. What Do You Mean By Standard Enthalpy Of Formation.
From www.facebook.com
Super Cheat Powers and a Chill Adventure Awaits! Anime Full Episode 1 What Do You Mean By Standard Enthalpy Of Formation A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from.. What Do You Mean By Standard Enthalpy Of Formation.
From www.youtube.com
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL) YouTube What Do You Mean By Standard Enthalpy Of Formation A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. The standard enthalpy of formation is a measure of the energy released or. What Do You Mean By Standard Enthalpy Of Formation.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube What Do You Mean By Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. The standard enthalpy of formation is a measure of the energy released or consumed when one. What Do You Mean By Standard Enthalpy Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies What Do You Mean By Standard Enthalpy Of Formation Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. What Do You Mean By Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free What Do You Mean By Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. What Do You Mean By Standard Enthalpy Of Formation.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics What Do You Mean By Standard Enthalpy Of Formation Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which. The standard enthalpy of formation. What Do You Mean By Standard Enthalpy Of Formation.
From www.youtube.com
CHEM 101 Using bond energies to calculate change in enthalpy for a What Do You Mean By Standard Enthalpy Of Formation Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy. What Do You Mean By Standard Enthalpy Of Formation.
From lessonlisttorpefying.z5.web.core.windows.net
What Is Enthalpy Quizlet Examples What Do You Mean By Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. Standard. What Do You Mean By Standard Enthalpy Of Formation.
From zeviernswenson.blogspot.com
Standard Enthalpy of Formation ZeviernSwenson What Do You Mean By Standard Enthalpy Of Formation Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation is defined as the enthalpy change when one mole. What Do You Mean By Standard Enthalpy Of Formation.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation What Do You Mean By Standard Enthalpy Of Formation The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For any substance at any particular temperature, we define the standard enthalpy of. What Do You Mean By Standard Enthalpy Of Formation.
From saylordotorg.github.io
Enthalpy What Do You Mean By Standard Enthalpy Of Formation A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change. What Do You Mean By Standard Enthalpy Of Formation.
From www.tes.com
Bond Enthalpy OCR A level Chemistry Teaching Resources What Do You Mean By Standard Enthalpy Of Formation Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which. 193 rows in chemistry and thermodynamics, the standard. What Do You Mean By Standard Enthalpy Of Formation.
From study.com
How to Draw & Label Enthalpy Diagrams Lesson What Do You Mean By Standard Enthalpy Of Formation A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of. What Do You Mean By Standard Enthalpy Of Formation.
From mungfali.com
Enthalpy Change Of Formation Equation What Do You Mean By Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy of formation is defined as the enthalpy change when one mole. What Do You Mean By Standard Enthalpy Of Formation.
From www.facebook.com
Super Cheat Powers and a Chill Adventure Awaits! Anime Full Episode 1 What Do You Mean By Standard Enthalpy Of Formation A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.. What Do You Mean By Standard Enthalpy Of Formation.
From www.doubtnut.com
[Bengali] (i) What do you mean by standard enthalpy of formation? (i What Do You Mean By Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction. What Do You Mean By Standard Enthalpy Of Formation.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube What Do You Mean By Standard Enthalpy Of Formation Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures. What Do You Mean By Standard Enthalpy Of Formation.
From www.youtube.com
Enthalpies of Reactions Using Average Bond Enthalpies Chemistry What Do You Mean By Standard Enthalpy Of Formation A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For any substance at any particular temperature, we define the. What Do You Mean By Standard Enthalpy Of Formation.
From mungfali.com
Standard Molar Enthalpy Of Formation Table What Do You Mean By Standard Enthalpy Of Formation The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation is defined as the enthalpy change when one mole. What Do You Mean By Standard Enthalpy Of Formation.
From www.facebook.com
Super Cheat Powers and a Chill Adventure Awaits! Anime Full Episode 1 What Do You Mean By Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. What Do You Mean By Standard Enthalpy Of Formation.
From www.youtube.com
Heat of Formation of MgO YouTube What Do You Mean By Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of. What Do You Mean By Standard Enthalpy Of Formation.
From schoolworkhelper.net
Standard Enthalpies of Formation Online Homework Help SchoolWorkHelper What Do You Mean By Standard Enthalpy Of Formation The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat. What Do You Mean By Standard Enthalpy Of Formation.
From lessonlistkilderkins.z22.web.core.windows.net
What Is Enthalpy Quizlet Examples What Do You Mean By Standard Enthalpy Of Formation Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. What Do You Mean By Standard Enthalpy Of Formation.
From learningdbkingswood.z13.web.core.windows.net
How To Work Out Enthalpy What Do You Mean By Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction. What Do You Mean By Standard Enthalpy Of Formation.
From priaxon.com
What Is Standard Enthalpy Of Formation Of Nh3 Gas Templates Printable What Do You Mean By Standard Enthalpy Of Formation Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation is defined as the enthalpy change when one mole. What Do You Mean By Standard Enthalpy Of Formation.