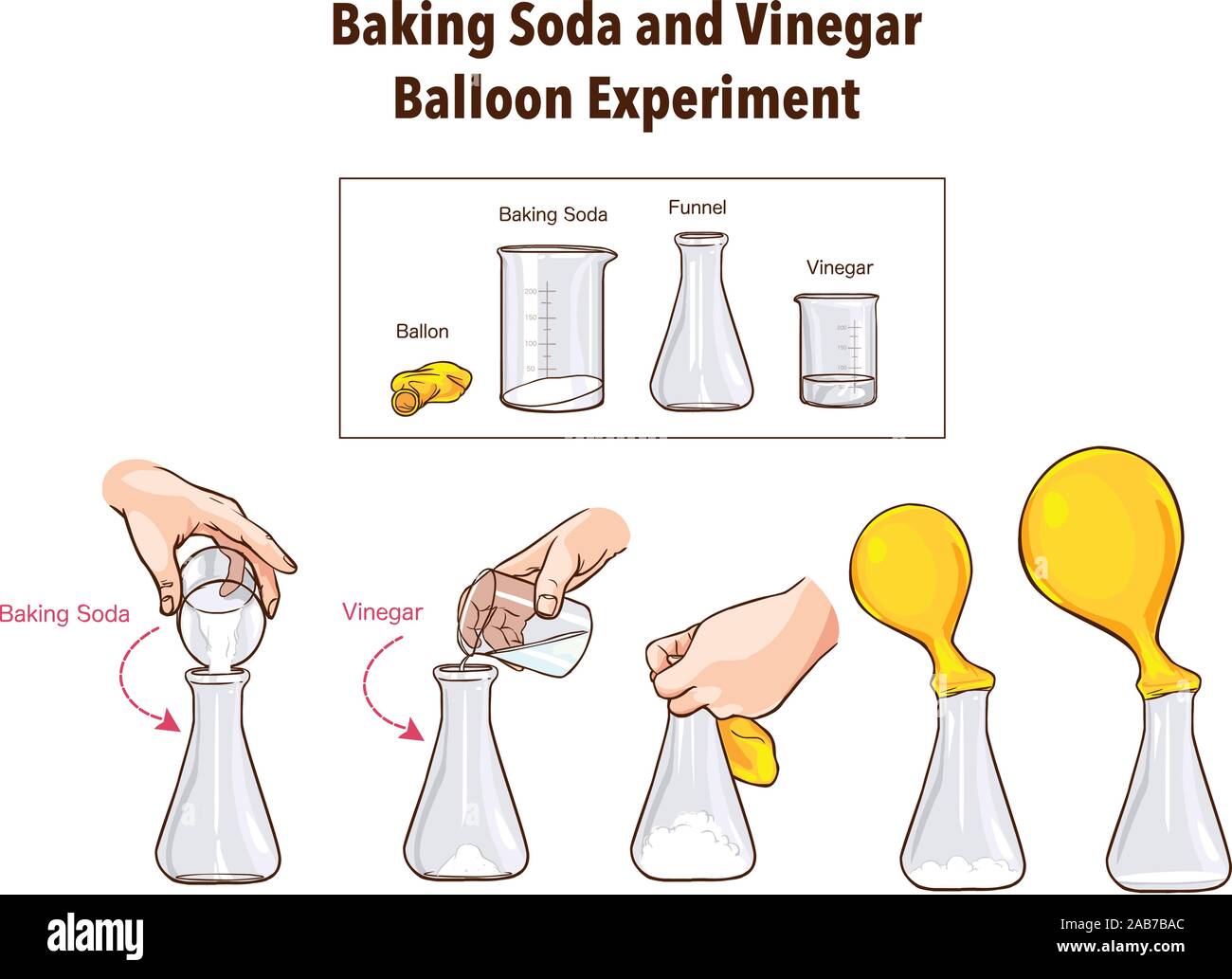
Baking Soda And Acid Chemical Reaction . The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. Baking soda is a powdered. The reaction proceeds in two steps. Mixing baking soda (sodium bicarbonate) and vinegar (acetic acid) causes a chemical reaction that produces a salt (sodium acetate) and. The water in the vinegar acts as a host where the base and acid react. This produces sodium acetate, water, and carbon dioxide gas. Baking soda and vinegar react to neutralise each other (. Sodium bicarbonate (baking soda) reacts with acetic acid (vinegar). During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). Baking soda is a base, and vinegar is an acid. Using the concept of stoichiometry, the amount of product that results from a chemical reaction can be predicted. In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and citric acid are combined. Vinegar isn't just an acid, it is an acid in water, which is important. In the process, several products are formed,. If you’ve ever made an erupting volcano model you most likely used the baking soda and vinegar reaction to make it erupt.
from lessonlibminimalist.z13.web.core.windows.net
The reaction proceeds in two steps. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. Baking soda is a base, and vinegar is an acid. Using the concept of stoichiometry, the amount of product that results from a chemical reaction can be predicted. Baking soda is a powdered. Mixing baking soda (sodium bicarbonate) and vinegar (acetic acid) causes a chemical reaction that produces a salt (sodium acetate) and. Vinegar isn't just an acid, it is an acid in water, which is important. In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and citric acid are combined. In the process, several products are formed,. This produces sodium acetate, water, and carbon dioxide gas.
Baking Soda And Vinegar Experiment Explained
Baking Soda And Acid Chemical Reaction During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). Vinegar isn't just an acid, it is an acid in water, which is important. The water in the vinegar acts as a host where the base and acid react. Baking soda and vinegar react to neutralise each other (. This produces sodium acetate, water, and carbon dioxide gas. In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and citric acid are combined. Mixing baking soda (sodium bicarbonate) and vinegar (acetic acid) causes a chemical reaction that produces a salt (sodium acetate) and. If you’ve ever made an erupting volcano model you most likely used the baking soda and vinegar reaction to make it erupt. Sodium bicarbonate (baking soda) reacts with acetic acid (vinegar). In the process, several products are formed,. Using the concept of stoichiometry, the amount of product that results from a chemical reaction can be predicted. The reaction proceeds in two steps. Baking soda is a powdered. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). Baking soda is a base, and vinegar is an acid.
From www.pinterest.com
A Simple Explanation the Difference Between Baking Soda & Baking Baking Soda And Acid Chemical Reaction Vinegar isn't just an acid, it is an acid in water, which is important. If you’ve ever made an erupting volcano model you most likely used the baking soda and vinegar reaction to make it erupt. Sodium bicarbonate (baking soda) reacts with acetic acid (vinegar). The water in the vinegar acts as a host where the base and acid react.. Baking Soda And Acid Chemical Reaction.
From www.youtube.com
Baking Soda and Vinegar Chemical Reaction (more balloons) Mister C Baking Soda And Acid Chemical Reaction Baking soda is a powdered. This produces sodium acetate, water, and carbon dioxide gas. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. Sodium. Baking Soda And Acid Chemical Reaction.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Baking Soda And Acid Chemical Reaction Vinegar isn't just an acid, it is an acid in water, which is important. Baking soda and vinegar react to neutralise each other (. The water in the vinegar acts as a host where the base and acid react. Sodium bicarbonate (baking soda) reacts with acetic acid (vinegar). In the process, several products are formed,. During the reaction, when the. Baking Soda And Acid Chemical Reaction.
From www.numerade.com
SOLVED The chemical equation for the reaction of baking soda (sodium Baking Soda And Acid Chemical Reaction The reaction proceeds in two steps. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. The water in the vinegar acts as a host. Baking Soda And Acid Chemical Reaction.
From www.pinterest.com
Vinegar Baking Soda Demonstrations Middle school chemistry, Middle Baking Soda And Acid Chemical Reaction Vinegar isn't just an acid, it is an acid in water, which is important. Baking soda is a powdered. Sodium bicarbonate (baking soda) reacts with acetic acid (vinegar). The reaction proceeds in two steps. Using the concept of stoichiometry, the amount of product that results from a chemical reaction can be predicted. Baking soda is a base, and vinegar is. Baking Soda And Acid Chemical Reaction.
From katierossparks.blogspot.com
Baking Soda Chemical Formula KatierosSparks Baking Soda And Acid Chemical Reaction During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). Baking soda is a base, and vinegar is an acid. Baking soda is a powdered. The reaction proceeds in two steps. If you’ve ever made an erupting volcano model you most likely used the baking soda and. Baking Soda And Acid Chemical Reaction.
From tikalon.com
Tikalon Blog by Dev Gualtieri Baking Soda And Acid Chemical Reaction Vinegar isn't just an acid, it is an acid in water, which is important. Using the concept of stoichiometry, the amount of product that results from a chemical reaction can be predicted. In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and citric acid are combined. In the process, several products are formed,. During the reaction,. Baking Soda And Acid Chemical Reaction.
From thomasyouthhouse.blogspot.com
Sodium Bicarbonate and Acetic Acid Balanced Chemical Equation Baking Soda And Acid Chemical Reaction Baking soda is a base, and vinegar is an acid. Using the concept of stoichiometry, the amount of product that results from a chemical reaction can be predicted. Sodium bicarbonate (baking soda) reacts with acetic acid (vinegar). Vinegar isn't just an acid, it is an acid in water, which is important. The reaction proceeds in two steps. Mixing baking soda. Baking Soda And Acid Chemical Reaction.
From www.alamy.com
Chemical reaction of phenolphthalein solution (Baking soda, Vinegar and Baking Soda And Acid Chemical Reaction The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and citric. Baking Soda And Acid Chemical Reaction.
From www.sciencephoto.com
Baking soda reacting with vinegar Stock Image A500/0648 Science Baking Soda And Acid Chemical Reaction Baking soda and vinegar react to neutralise each other (. Sodium bicarbonate (baking soda) reacts with acetic acid (vinegar). Baking soda is a powdered. If you’ve ever made an erupting volcano model you most likely used the baking soda and vinegar reaction to make it erupt. In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and. Baking Soda And Acid Chemical Reaction.
From signalticket9.pythonanywhere.com
Marvelous Vinegar Plus Baking Soda Chemical Equation Balanced For And Baking Soda And Acid Chemical Reaction Baking soda and vinegar react to neutralise each other (. Sodium bicarbonate (baking soda) reacts with acetic acid (vinegar). This produces sodium acetate, water, and carbon dioxide gas. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). Vinegar isn't just an acid, it is an acid. Baking Soda And Acid Chemical Reaction.
From chemistry291.blogspot.com
Baking Soda and Vinegar Chemical Reaction ExplanationNaHCO3 + CH3COOH Baking Soda And Acid Chemical Reaction The reaction proceeds in two steps. Baking soda is a base, and vinegar is an acid. Mixing baking soda (sodium bicarbonate) and vinegar (acetic acid) causes a chemical reaction that produces a salt (sodium acetate) and. The water in the vinegar acts as a host where the base and acid react. Baking soda is a powdered. The overall chemical reaction. Baking Soda And Acid Chemical Reaction.
From chemistry291.blogspot.com
Baking Soda and Vinegar Chemical Reaction ExplanationNaHCO3 + CH3COOH Baking Soda And Acid Chemical Reaction Using the concept of stoichiometry, the amount of product that results from a chemical reaction can be predicted. Baking soda is a powdered. Baking soda and vinegar react to neutralise each other (. The reaction proceeds in two steps. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the. Baking Soda And Acid Chemical Reaction.
From printables.it.com
Baking Soda Science Printables Chemical Reaction Free Printable Download Baking Soda And Acid Chemical Reaction Sodium bicarbonate (baking soda) reacts with acetic acid (vinegar). Mixing baking soda (sodium bicarbonate) and vinegar (acetic acid) causes a chemical reaction that produces a salt (sodium acetate) and. Using the concept of stoichiometry, the amount of product that results from a chemical reaction can be predicted. Baking soda and vinegar react to neutralise each other (. The reaction proceeds. Baking Soda And Acid Chemical Reaction.
From issuu.com
Endothermic reactions citric acide and baking soda by Fourier Education Baking Soda And Acid Chemical Reaction The water in the vinegar acts as a host where the base and acid react. The reaction proceeds in two steps. In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and citric acid are combined. Mixing baking soda (sodium bicarbonate) and vinegar (acetic acid) causes a chemical reaction that produces a salt (sodium acetate) and. In. Baking Soda And Acid Chemical Reaction.
From www.tessshebaylo.com
Chemical Equation For Baking Soda Citric Acid And Water Tessshebaylo Baking Soda And Acid Chemical Reaction Baking soda is a base, and vinegar is an acid. This produces sodium acetate, water, and carbon dioxide gas. In the process, several products are formed,. Vinegar isn't just an acid, it is an acid in water, which is important. Baking soda and vinegar react to neutralise each other (. The reaction proceeds in two steps. Sodium bicarbonate (baking soda). Baking Soda And Acid Chemical Reaction.
From www.youtube.com
Chemical Reaction Of Baking Soda And Vinegar (Sodium Bicarbonate And Baking Soda And Acid Chemical Reaction If you’ve ever made an erupting volcano model you most likely used the baking soda and vinegar reaction to make it erupt. In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and citric acid are combined. The reaction proceeds in two steps. The water in the vinegar acts as a host where the base and acid. Baking Soda And Acid Chemical Reaction.
From www.ingridscience.ca
Baking soda and vinegar ingridscience.ca Baking Soda And Acid Chemical Reaction In the process, several products are formed,. Baking soda is a base, and vinegar is an acid. Baking soda is a powdered. The water in the vinegar acts as a host where the base and acid react. Using the concept of stoichiometry, the amount of product that results from a chemical reaction can be predicted. Baking soda and vinegar react. Baking Soda And Acid Chemical Reaction.
From www.youtube.com
Citric acid and Baking Soda Reaction YouTube Baking Soda And Acid Chemical Reaction In the process, several products are formed,. The reaction proceeds in two steps. If you’ve ever made an erupting volcano model you most likely used the baking soda and vinegar reaction to make it erupt. In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and citric acid are combined. During the reaction, when the baking soda. Baking Soda And Acid Chemical Reaction.
From www.pinterest.com
Citric Acid and Baking Soda Reaction Little Bins for Little Hands Baking Soda And Acid Chemical Reaction Vinegar isn't just an acid, it is an acid in water, which is important. Baking soda is a powdered. Sodium bicarbonate (baking soda) reacts with acetic acid (vinegar). If you’ve ever made an erupting volcano model you most likely used the baking soda and vinegar reaction to make it erupt. The overall chemical reaction between baking soda (sodium bicarbonate) and. Baking Soda And Acid Chemical Reaction.
From www.youtube.com
Acid Base Neutralization Reaction (Baking Soda and Sulfuric Acid) YouTube Baking Soda And Acid Chemical Reaction Baking soda and vinegar react to neutralise each other (. If you’ve ever made an erupting volcano model you most likely used the baking soda and vinegar reaction to make it erupt. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). This produces sodium acetate, water,. Baking Soda And Acid Chemical Reaction.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Baking Soda And Acid Chemical Reaction Sodium bicarbonate (baking soda) reacts with acetic acid (vinegar). In the process, several products are formed,. Baking soda and vinegar react to neutralise each other (. Using the concept of stoichiometry, the amount of product that results from a chemical reaction can be predicted. Baking soda is a powdered. If you’ve ever made an erupting volcano model you most likely. Baking Soda And Acid Chemical Reaction.
From littlebinsforlittlehands.com
Citric Acid and Baking Soda Reaction Little Bins for Little Hands Baking Soda And Acid Chemical Reaction During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). Mixing baking soda (sodium bicarbonate) and vinegar (acetic acid) causes a chemical reaction that produces a salt (sodium acetate) and. The reaction proceeds in two steps. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar. Baking Soda And Acid Chemical Reaction.
From www.youtube.com
Chemical reaction mixing baking soda and vinegar YouTube Baking Soda And Acid Chemical Reaction In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and citric acid are combined. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). In the process, several products are formed,. The reaction proceeds in two steps. Baking soda is a base, and. Baking Soda And Acid Chemical Reaction.
From www.youtube.com
Signs of Chemical Reactions Lab Citric Acid and Baking Soda Temp Change Baking Soda And Acid Chemical Reaction Baking soda and vinegar react to neutralise each other (. In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and citric acid are combined. Mixing baking soda (sodium bicarbonate) and vinegar (acetic acid) causes a chemical reaction that produces a salt (sodium acetate) and. This produces sodium acetate, water, and carbon dioxide gas. Vinegar isn't just. Baking Soda And Acid Chemical Reaction.
From www.youtube.com
Citric acid + baking soda + water reaction YouTube Baking Soda And Acid Chemical Reaction Mixing baking soda (sodium bicarbonate) and vinegar (acetic acid) causes a chemical reaction that produces a salt (sodium acetate) and. The water in the vinegar acts as a host where the base and acid react. Vinegar isn't just an acid, it is an acid in water, which is important. Baking soda and vinegar react to neutralise each other (. The. Baking Soda And Acid Chemical Reaction.
From lessonlibminimalist.z13.web.core.windows.net
Baking Soda And Vinegar Experiment Explained Baking Soda And Acid Chemical Reaction Mixing baking soda (sodium bicarbonate) and vinegar (acetic acid) causes a chemical reaction that produces a salt (sodium acetate) and. Using the concept of stoichiometry, the amount of product that results from a chemical reaction can be predicted. In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and citric acid are combined. Vinegar isn't just an. Baking Soda And Acid Chemical Reaction.
From bakingsodaandvinegarbonding.weebly.com
Bonding Process Baking Soda And Acid Chemical Reaction In the process, several products are formed,. Mixing baking soda (sodium bicarbonate) and vinegar (acetic acid) causes a chemical reaction that produces a salt (sodium acetate) and. Vinegar isn't just an acid, it is an acid in water, which is important. In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and citric acid are combined. If. Baking Soda And Acid Chemical Reaction.
From sciencenotes.org
Chemical Equation for Baking Soda and Vinegar Reaction Baking Soda And Acid Chemical Reaction In the process, several products are formed,. Using the concept of stoichiometry, the amount of product that results from a chemical reaction can be predicted. The reaction proceeds in two steps. The water in the vinegar acts as a host where the base and acid react. Baking soda is a base, and vinegar is an acid. Baking soda and vinegar. Baking Soda And Acid Chemical Reaction.
From www.tessshebaylo.com
Chemical Equation For Baking Soda Citric Acid And Water Tessshebaylo Baking Soda And Acid Chemical Reaction Vinegar isn't just an acid, it is an acid in water, which is important. In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and citric acid are combined. Baking soda is a base, and vinegar is an acid. Mixing baking soda (sodium bicarbonate) and vinegar (acetic acid) causes a chemical reaction that produces a salt (sodium. Baking Soda And Acid Chemical Reaction.
From www.alamy.com
Chemical Reaction of Baking Soda (sodium bicarbonate) and Vinegar Baking Soda And Acid Chemical Reaction Using the concept of stoichiometry, the amount of product that results from a chemical reaction can be predicted. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). This produces sodium acetate, water, and carbon dioxide gas. In this chemical reaction, baking soda (also known to chemists. Baking Soda And Acid Chemical Reaction.
From www.youtube.com
Baking Soda and Vinegar Chemical Reaction (EXPERIMENT EXPLAINED) YouTube Baking Soda And Acid Chemical Reaction Baking soda and vinegar react to neutralise each other (. The reaction proceeds in two steps. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate. Baking Soda And Acid Chemical Reaction.
From simpleathome.com
Chemical Reactions Acids and Bases • Simple At Home Baking Soda And Acid Chemical Reaction Baking soda is a base, and vinegar is an acid. The reaction proceeds in two steps. Sodium bicarbonate (baking soda) reacts with acetic acid (vinegar). Baking soda is a powdered. The water in the vinegar acts as a host where the base and acid react. In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and citric. Baking Soda And Acid Chemical Reaction.
From www.youtube.com
Type of Reaction for Baking Soda and Vinegar ( NaHCO3 + CH3COOH) YouTube Baking Soda And Acid Chemical Reaction If you’ve ever made an erupting volcano model you most likely used the baking soda and vinegar reaction to make it erupt. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas,. Baking Soda And Acid Chemical Reaction.
From chemistry291.blogspot.com
Baking Soda and Vinegar Chemical Reaction ExplanationNaHCO3 + CH3COOH Baking Soda And Acid Chemical Reaction In this chemical reaction, baking soda (also known to chemists as sodium bicarbonate) and citric acid are combined. Baking soda is a base, and vinegar is an acid. Baking soda and vinegar react to neutralise each other (. If you’ve ever made an erupting volcano model you most likely used the baking soda and vinegar reaction to make it erupt.. Baking Soda And Acid Chemical Reaction.