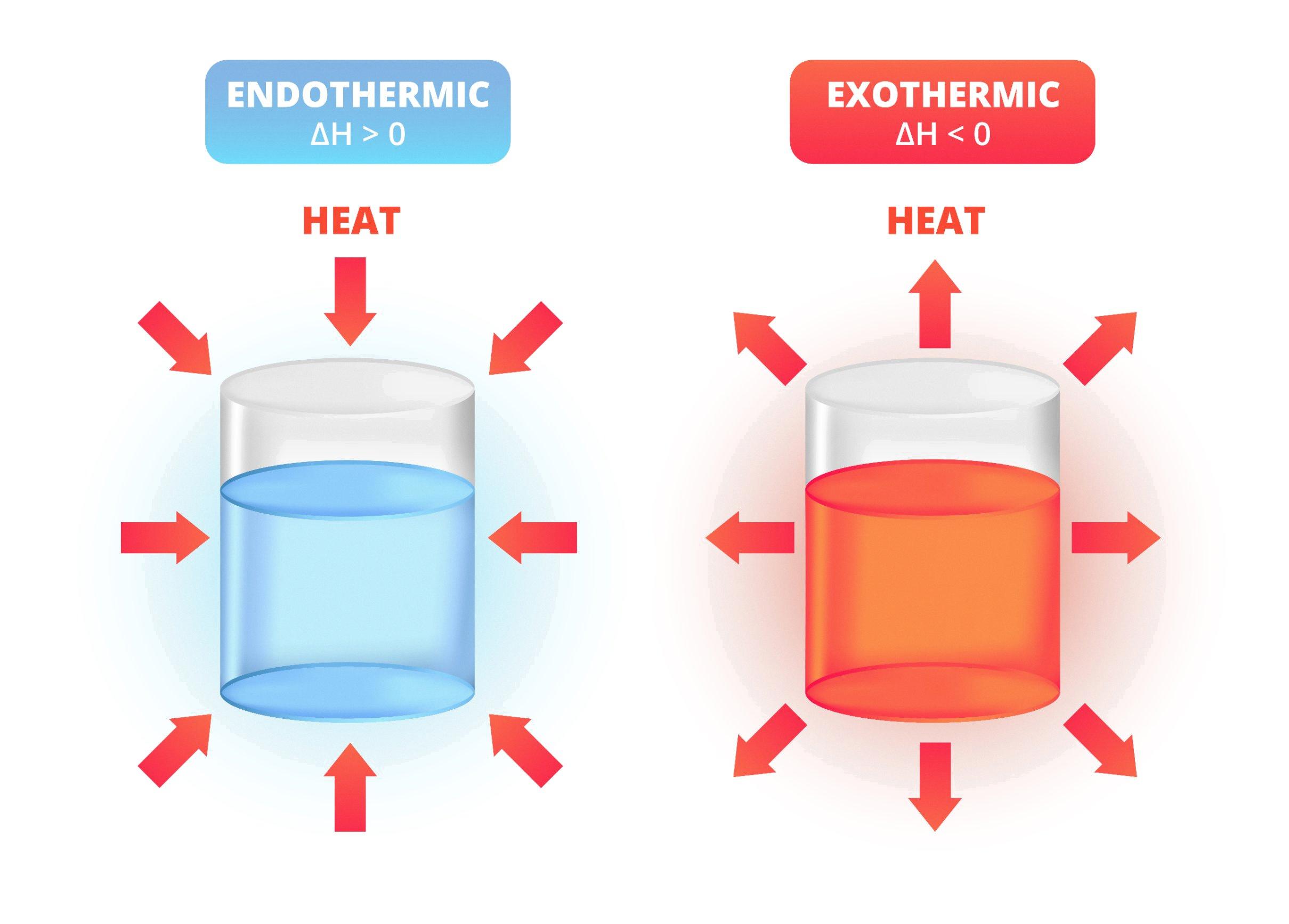
Endothermic Heat Of Solution . The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. From your three runs determine an average δ hsoln for your salt. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved when a solute is. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Determination of a heat of solution. Also calculate the estimated standard deviation and the 95% confidence. The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water.
from h-o-m-e.org
The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved when a solute is. The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Determination of a heat of solution. Also calculate the estimated standard deviation and the 95% confidence. From your three runs determine an average δ hsoln for your salt.
Endothermic Reactions The Science Behind Temperature Change
Endothermic Heat Of Solution The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. From your three runs determine an average δ hsoln for your salt. Determination of a heat of solution. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved when a solute is. Also calculate the estimated standard deviation and the 95% confidence.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Heat Of Solution Also calculate the estimated standard deviation and the 95% confidence. Determination of a heat of solution. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved. Endothermic Heat Of Solution.
From www.chemistrystudent.com
Enthalpy of Solution (Alevel) ChemistryStudent Endothermic Heat Of Solution The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. From your three runs determine an average δ hsoln for your salt.. Endothermic Heat Of Solution.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Heat Of Solution The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. From your three runs determine an average δ hsoln for your salt. Determination of a heat of solution. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed. Endothermic Heat Of Solution.
From www.coursehero.com
[Solved] Chemistry Part B In an endothermic reaction, is the energy of Endothermic Heat Of Solution The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. From your three runs determine an average δ hsoln for your salt. Determination of. Endothermic Heat Of Solution.
From www.numerade.com
SOLVEDGive one example of each a salt whose heat of solution is Endothermic Heat Of Solution From your three runs determine an average δ hsoln for your salt. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved when a solute is. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at.. Endothermic Heat Of Solution.
From www.youtube.com
Endothermic and exothermic reactions. Enthalpy YouTube Endothermic Heat Of Solution The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved when a solute is. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Determination of a heat of solution. The enthalpy of solution (δh soln). Endothermic Heat Of Solution.
From improove.in
Experiment 16 Heat generation during reaction Improove Endothermic Heat Of Solution The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved when a solute is. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. The molar heat of solution (δhsoln) of a substance is. Endothermic Heat Of Solution.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Endothermic Heat Of Solution From your three runs determine an average δ hsoln for your salt. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved when a solute is. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in. Endothermic Heat Of Solution.
From slideplayer.com
The Molecular Nature of Matter and Change ppt download Endothermic Heat Of Solution The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. From your three runs determine an average δ hsoln for your salt. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved when a. Endothermic Heat Of Solution.
From slideplayer.com
Solutions. ppt download Endothermic Heat Of Solution The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved when a solute is. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. The molar heat of solution (δhsoln) of a substance is. Endothermic Heat Of Solution.
From slideplayer.com
Chapter 5 Chemical Reactions and Quantities ppt download Endothermic Heat Of Solution From your three runs determine an average δ hsoln for your salt. Determination of a heat of solution. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed. Endothermic Heat Of Solution.
From www.sliderbase.com
Formation of Solutions Presentation Chemistry Endothermic Heat Of Solution Also calculate the estimated standard deviation and the 95% confidence. From your three runs determine an average δ hsoln for your salt. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of. Endothermic Heat Of Solution.
From elecdiags.com
The Significance and Use of Endothermic Reaction Profile Diagrams in Endothermic Heat Of Solution The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. Determination of a heat of solution. Also calculate the estimated standard deviation and the 95% confidence. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute. Endothermic Heat Of Solution.
From www.chemistrystudent.com
Enthalpy of Solution (Alevel) ChemistryStudent Endothermic Heat Of Solution Determination of a heat of solution. Also calculate the estimated standard deviation and the 95% confidence. The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that. Endothermic Heat Of Solution.
From mareeromana.blogspot.com
12+ Endothermic Enthalpy Diagram MareeRomana Endothermic Heat Of Solution From your three runs determine an average δ hsoln for your salt. Also calculate the estimated standard deviation and the 95% confidence. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved when a solute is. The enthalpy of solution (δh soln) is the heat released or. Endothermic Heat Of Solution.
From slideplayer.com
Solutions. ppt download Endothermic Heat Of Solution The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Determination of a heat of solution. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved when a solute is. The molar heat of. Endothermic Heat Of Solution.
From www.numerade.com
Is the solution process exothermic (energy released, temperature Endothermic Heat Of Solution The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Determination of a heat of solution. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Also calculate the estimated standard deviation and the 95% confidence.. Endothermic Heat Of Solution.
From www.chemistrylearner.com
Heat (Enthalpy) of Solution Definition, Formula, & Problems Endothermic Heat Of Solution The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. From your three runs determine an average δ hsoln for your salt. Also calculate the estimated standard deviation and the 95% confidence. The enthalpy change of solution refers to the amount of heat that is. Endothermic Heat Of Solution.
From www.vecteezy.com
Enthalpy Endothermic versus Endothermic biochemistry vector Endothermic Heat Of Solution The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Also calculate the estimated standard deviation and the 95% confidence. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Determination of a heat of solution.. Endothermic Heat Of Solution.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Heat Of Solution The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. Determination of a heat of solution. The enthalpy change of solution refers. Endothermic Heat Of Solution.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Heat Of Solution The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. From your three runs determine an average δ hsoln for your salt. The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The enthalpy. Endothermic Heat Of Solution.
From www.chemistrysteps.com
Energy and Organic Chemistry Reactions Chemistry Steps Endothermic Heat Of Solution Determination of a heat of solution. From your three runs determine an average δ hsoln for your salt. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved when a solute is. The molar heat of solution (δhsoln) of a substance is the heat absorbed or released. Endothermic Heat Of Solution.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Endothermic Heat Of Solution The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. Also calculate the estimated standard deviation and the 95% confidence. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The enthalpy of solution,. Endothermic Heat Of Solution.
From www.expii.com
Energy Diagram — Overview & Parts Expii Endothermic Heat Of Solution From your three runs determine an average δ hsoln for your salt. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Also calculate the estimated standard deviation and the 95% confidence. The molar heat of solution (δhsoln) of a substance is the heat absorbed or released. Endothermic Heat Of Solution.
From www.slideserve.com
PPT Chapter 7 Solutions and Colloids PowerPoint Presentation, free Endothermic Heat Of Solution The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved when a solute is. Also calculate the estimated standard deviation and the 95% confidence.. Endothermic Heat Of Solution.
From slideplayer.com
States of Matter & Energy ppt download Endothermic Heat Of Solution The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved when a solute is. Also calculate the estimated standard deviation and the 95% confidence.. Endothermic Heat Of Solution.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Heat Of Solution Also calculate the estimated standard deviation and the 95% confidence. The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Determination of. Endothermic Heat Of Solution.
From slideplayer.com
, Thermodynamics and Equilibrium ppt download Endothermic Heat Of Solution Determination of a heat of solution. From your three runs determine an average δ hsoln for your salt. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in. Endothermic Heat Of Solution.
From slideplayer.com
Solutions Part III IMF, Energetics of Solutions, Henry’s Law Much of Endothermic Heat Of Solution From your three runs determine an average δ hsoln for your salt. Determination of a heat of solution. The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The enthalpy change of solution refers to the amount of heat that is released or absorbed during. Endothermic Heat Of Solution.
From www.studypool.com
SOLUTION Energy changes in chemical reactions activation energy Endothermic Heat Of Solution The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. Also calculate the estimated standard deviation and the 95% confidence. The enthalpy of solution. Endothermic Heat Of Solution.
From www.slideserve.com
PPT Heat from Reactions PowerPoint Presentation, free download ID Endothermic Heat Of Solution Determination of a heat of solution. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Also calculate the estimated standard deviation and the 95% confidence. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at.. Endothermic Heat Of Solution.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Endothermic Heat Of Solution The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Also calculate the estimated standard deviation and the 95% confidence. Determination of a heat of solution. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at.. Endothermic Heat Of Solution.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Heat Of Solution The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. Determination of a heat of solution. From your three runs determine an average δ hsoln for your salt. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of. Endothermic Heat Of Solution.
From www.studocu.com
CHEM212Chapter 13 Chapter 13 Heat of Solutions, Solubility of Gases Endothermic Heat Of Solution Also calculate the estimated standard deviation and the 95% confidence. From your three runs determine an average δ hsoln for your salt. Determination of a heat of solution. The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The enthalpy change of solution refers to. Endothermic Heat Of Solution.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Heat Of Solution The molar heat of solution (δhsoln) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The enthalpy of solution, also known as the heat of solution, is the total amount of heat energy that is absorbed or evolved when a solute is. Also calculate the estimated standard deviation and the. Endothermic Heat Of Solution.