Paraffin Candle Wax Equation . Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where some wax is oxidized to elemental c (soot) and water. Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. Each component of candle wax has its own particular combustion reaction. This reaction turns the solid wax into invisible carbon dioxide gas. In this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o2(g). This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and. However, since paraffin is made up of saturated. Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. The equation may look as follows:
from www.numerade.com
Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. In this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o2(g). Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where some wax is oxidized to elemental c (soot) and water. Each component of candle wax has its own particular combustion reaction. Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. However, since paraffin is made up of saturated. The equation may look as follows: This reaction turns the solid wax into invisible carbon dioxide gas. This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and.
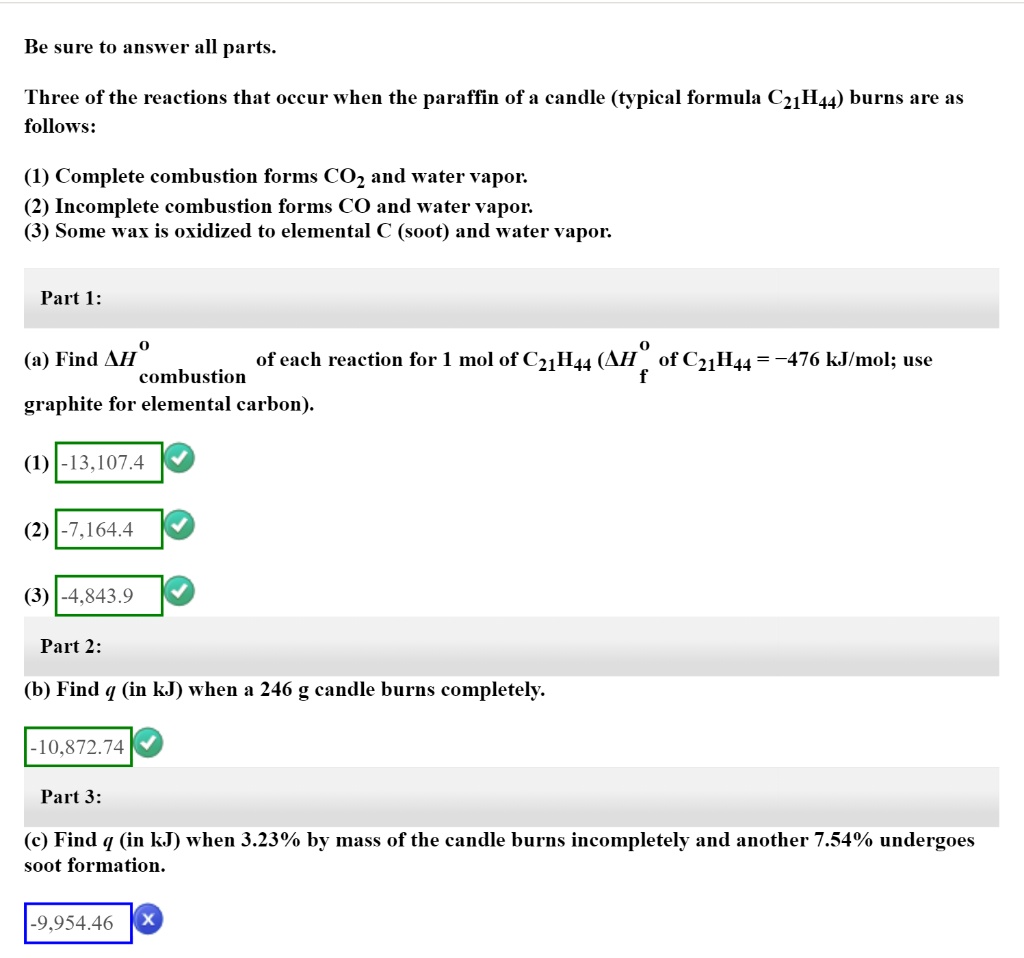
SOLVED Be sure to answer all parts. Three of the reactions that occur
Paraffin Candle Wax Equation However, since paraffin is made up of saturated. This reaction turns the solid wax into invisible carbon dioxide gas. However, since paraffin is made up of saturated. The equation may look as follows: In this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o2(g). This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and. Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where some wax is oxidized to elemental c (soot) and water. Each component of candle wax has its own particular combustion reaction. Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$.
From ar.inspiredpencil.com
Paraffin Wax Structure Paraffin Candle Wax Equation The equation may look as follows: In this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o2(g). Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. Each component of candle wax has its. Paraffin Candle Wax Equation.
From www.researchgate.net
Treated paraffin wax by emulsified PD90 Download Scientific Diagram Paraffin Candle Wax Equation Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where some wax is oxidized to elemental c (soot) and water. Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon. Paraffin Candle Wax Equation.
From www.researchgate.net
Properties of paraffin candle wax from the literature. Download Table Paraffin Candle Wax Equation This reaction turns the solid wax into invisible carbon dioxide gas. The equation may look as follows: Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. However, since paraffin is made up of saturated. Find. Paraffin Candle Wax Equation.
From www.youtube.com
How To Make A Paraffin Wax Candle Candle Making For Beginners YouTube Paraffin Candle Wax Equation Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. The equation may look as follows: Each component of candle wax has its own particular combustion reaction. This equation shows that when one molecule of candle. Paraffin Candle Wax Equation.
From www.pinterest.jp
CHOOSE soy wax candles versus paraffin ones...here is a chart Paraffin Candle Wax Equation This reaction turns the solid wax into invisible carbon dioxide gas. The equation may look as follows: Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where some wax is oxidized to elemental c. Paraffin Candle Wax Equation.
From www.chegg.com
Solved 2. Paraffin wax is the main ingredient in candles. Paraffin Candle Wax Equation This reaction turns the solid wax into invisible carbon dioxide gas. Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where some wax is oxidized to elemental c (soot) and water. However, since paraffin is made up of saturated. The equation may look as follows: In this investigation, students will study the rate of burning. Paraffin Candle Wax Equation.
From www.youtube.com
How to Balance C25H52 + O2 = CO2 + H2O (Wax + Oxygen gas ) YouTube Paraffin Candle Wax Equation Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where some wax is oxidized to elemental c (soot) and water. This. Paraffin Candle Wax Equation.
From www.researchgate.net
DSC curves of the paraffin wax PCM (PWPCM) Download Scientific Diagram Paraffin Candle Wax Equation Each component of candle wax has its own particular combustion reaction. Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. The equation may look as follows: This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon. Paraffin Candle Wax Equation.
From www.slideserve.com
PPT Candles are made out of Paraffin Wax. The chemical formula for Paraffin Candle Wax Equation Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and. Each component of candle wax has its own particular combustion reaction. This reaction turns the. Paraffin Candle Wax Equation.
From www.numerade.com
SOLVED Paraffin, a wax used to make candles, has a formula of C25H52 Paraffin Candle Wax Equation The equation may look as follows: Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where some wax is oxidized to elemental c (soot) and water. This reaction turns the solid wax into invisible carbon dioxide gas. However, since paraffin is made up of saturated. This equation shows that when one molecule of candle wax. Paraffin Candle Wax Equation.
From www.numerade.com
SOLVED Paraffin, a wax used to make candles, has a formula of C25H52 Paraffin Candle Wax Equation However, since paraffin is made up of saturated. The equation may look as follows: Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where some wax is oxidized to elemental c (soot) and water. Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. This equation shows that when one molecule of. Paraffin Candle Wax Equation.
From www.pinterest.com
Pin on teaching Matter and Energy Paraffin Candle Wax Equation Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and. The equation may look as follows: However, since paraffin is made up of saturated. Each component of candle wax has its own particular. Paraffin Candle Wax Equation.
From www.numerade.com
SOLVED 1. Paraffin wax, used in candles, has a molecular formula of Paraffin Candle Wax Equation The equation may look as follows: This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and. Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where some wax. Paraffin Candle Wax Equation.
From ar.inspiredpencil.com
Paraffin Wax Structure Paraffin Candle Wax Equation In this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o2(g). However, since paraffin is made up of saturated. The equation may look as follows: This equation shows that when one molecule of candle wax reacts with. Paraffin Candle Wax Equation.
From www.candlescience.com
Candle Wax Soy, Paraffin, Coconut, and Beeswax for Candle Making Paraffin Candle Wax Equation The equation may look as follows: Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where some wax is oxidized to elemental c (soot) and water. Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the. Paraffin Candle Wax Equation.
From shaylakruwyang.blogspot.com
Chemical Properties of Candle Wax ShaylakruwYang Paraffin Candle Wax Equation Each component of candle wax has its own particular combustion reaction. Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where. Paraffin Candle Wax Equation.
From www.youtube.com
HOW TO MAKE SCENTED CANDLES USING PARAFFIN WAX YouTube Paraffin Candle Wax Equation Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. In this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o2(g). The equation may look as. Paraffin Candle Wax Equation.
From junda999.en.made-in-china.com
Paraffin Wax Chemical Formula Wholesale Paraffin Candle Wax China Paraffin Candle Wax Equation Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and. Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. Find the. Paraffin Candle Wax Equation.
From www.researchgate.net
Properties of paraffin candle wax from the literature. Download Table Paraffin Candle Wax Equation This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and. Each component of candle wax has its own particular combustion reaction. Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. The equation may look. Paraffin Candle Wax Equation.
From devan-bloghuang.blogspot.com
Chemical Properties of Candle Wax Paraffin Candle Wax Equation Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where some wax is oxidized to elemental c (soot) and water. This reaction turns the solid wax into invisible carbon dioxide gas. This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and.. Paraffin Candle Wax Equation.
From www.slideserve.com
PPT Determining the Molar Heat of Combustion for Paraffin PowerPoint Paraffin Candle Wax Equation This reaction turns the solid wax into invisible carbon dioxide gas. Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and. The equation may look as follows: Find the heat of combustion for. Paraffin Candle Wax Equation.
From www.scribd.com
Basic Paraffin Wax Candle Making Instructions PDF Candle Wax Paraffin Candle Wax Equation The equation may look as follows: Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. In this investigation, students will study the rate of burning of a candle as a function of the mass of. Paraffin Candle Wax Equation.
From answerhappy.com
Be sure to answer all parts. Three of the reactions that occur when the Paraffin Candle Wax Equation However, since paraffin is made up of saturated. The equation may look as follows: Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. This reaction turns the solid wax into invisible carbon dioxide gas. This equation shows that when one molecule of candle wax reacts with 38 molecules. Paraffin Candle Wax Equation.
From www.researchgate.net
Chemical structure of paraffin wax (A), mxylene (B) and nheptane (C Paraffin Candle Wax Equation This reaction turns the solid wax into invisible carbon dioxide gas. This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and. In this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a. Paraffin Candle Wax Equation.
From hebeirunyo1816.en.made-in-china.com
Fully Refined Paraffin Wax 5860 for Carved Candles Chemical Formula Paraffin Candle Wax Equation This reaction turns the solid wax into invisible carbon dioxide gas. In this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o2(g). This equation shows that when one molecule of candle wax reacts with 38 molecules of. Paraffin Candle Wax Equation.
From www.differencebetween.com
Difference Between Paraffin Wax and Candle Wax Compare the Difference Paraffin Candle Wax Equation This reaction turns the solid wax into invisible carbon dioxide gas. The equation may look as follows: This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and. Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. Each component of candle wax has. Paraffin Candle Wax Equation.
From studylib.net
Molar Enthalpy of Paraffin Paraffin Candle Wax Equation This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and. Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. However, since. Paraffin Candle Wax Equation.
From ar.inspiredpencil.com
Paraffin Wax Structure Paraffin Candle Wax Equation However, since paraffin is made up of saturated. The equation may look as follows: Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. Each component of candle wax has its own particular combustion reaction. In this investigation, students will study the rate of burning of a candle as a function of the mass of the candle. Paraffin Candle Wax Equation.
From junda999.en.made-in-china.com
Paraffin Wax Chemical Formula China Candle Wax and Paraffin Wax Suppliers Paraffin Candle Wax Equation The equation may look as follows: This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and. Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence. Paraffin Candle Wax Equation.
From www.numerade.com
SOLVED Be sure to answer all parts. Three of the reactions that occur Paraffin Candle Wax Equation Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where some wax is oxidized to elemental c (soot) and water. Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. However, since paraffin is made up of saturated. In this investigation, students will study the rate of burning of a candle as. Paraffin Candle Wax Equation.
From www.thesprucecrafts.com
Paraffin Wax for Candle Making Paraffin Candle Wax Equation This reaction turns the solid wax into invisible carbon dioxide gas. This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and. The equation may look as follows: Each component of candle wax has its own particular combustion reaction. In this investigation, students will study the rate. Paraffin Candle Wax Equation.
From junda999.en.made-in-china.com
Kunlun Fully Refined IndustrialGrade Paraffin Wax Chemical Formula for Paraffin Candle Wax Equation However, since paraffin is made up of saturated. This reaction turns the solid wax into invisible carbon dioxide gas. The equation may look as follows: This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and. In this investigation, students will study the rate of burning of. Paraffin Candle Wax Equation.
From www.deviantart.com
Chemical Formula Of Wax by finabinnieiw9 on DeviantArt Paraffin Candle Wax Equation In this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of o2(g). Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where some wax is oxidized to elemental c (soot) and water. Candle. Paraffin Candle Wax Equation.
From 3dead2053119ef37.en.made-in-china.com
for Chemical Formula Paraffin Wax Carved Candles Wax China Wax and Paraffin Candle Wax Equation This equation shows that when one molecule of candle wax reacts with 38 molecules of oxygen, it produces 25 molecules of carbon dioxide and. Find the heat of combustion for 1 mol of $\ce {c21h44}$ in the reaction where some wax is oxidized to elemental c (soot) and water. In this investigation, students will study the rate of burning of. Paraffin Candle Wax Equation.
From www.researchgate.net
Properties of paraffin candle wax from the literature. Download Table Paraffin Candle Wax Equation Candles these days are commonly made out of paraffine, which is basically $\mathrm{c_{many}h_{many}}$. This reaction turns the solid wax into invisible carbon dioxide gas. The equation may look as follows: Candle wax is transformed by a combustion reaction involving the heat of the flame, plus the presence of oxygen and fuel. However, since paraffin is made up of saturated. Find. Paraffin Candle Wax Equation.