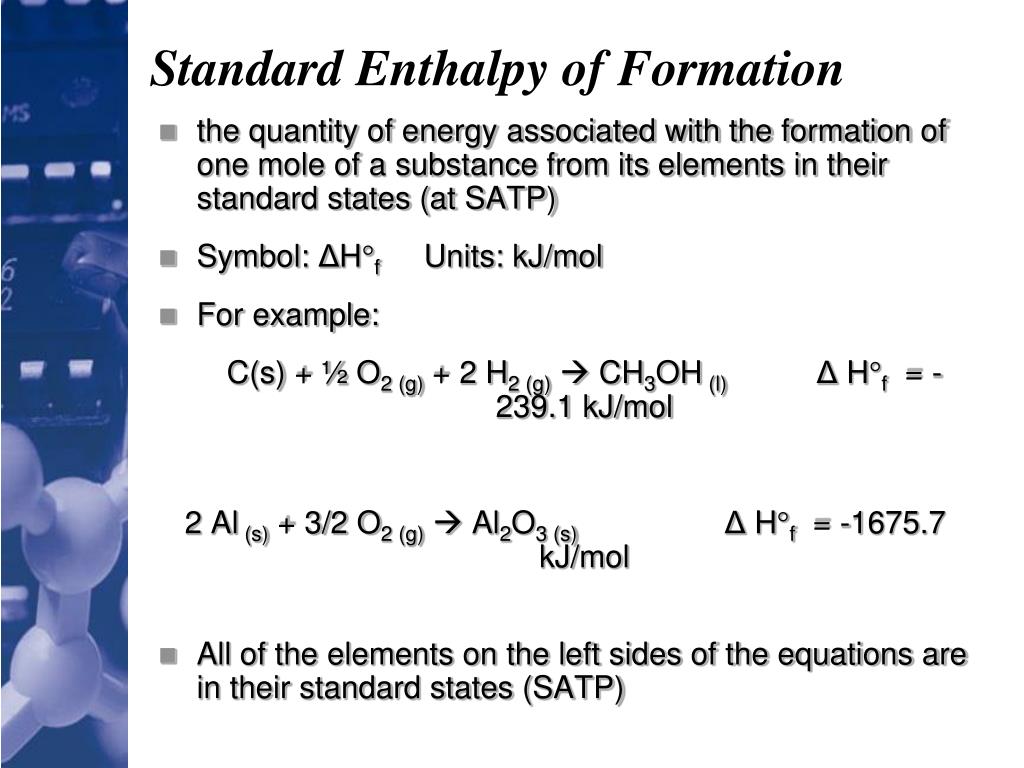
Standard Enthalpy Of Formation N2O4 Gas . The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including.
from www.slideserve.com
The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). The standard enthalpy of formation of any element in its standard state is zero by definition.
PPT Standard Enthalpies of Formation PowerPoint Presentation, free
Standard Enthalpy Of Formation N2O4 Gas Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas Heat Of Formation Standard Enthalpy Of Formation N2O4 Gas The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). For example, although. Standard Enthalpy Of Formation N2O4 Gas.
From www.toppr.com
The standard heat of formation of NO2(g) and N2O4(g) are 8.0 and 4.0 Standard Enthalpy Of Formation N2O4 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation. Standard Enthalpy Of Formation N2O4 Gas.
From www.toppr.com
The standard heat of formation of NO2(g) and N2O4(g) are 8.0 and 4.0 Standard Enthalpy Of Formation N2O4 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). 193 rows in. Standard Enthalpy Of Formation N2O4 Gas.
From mungfali.com
Enthalpies Of Formation Table Standard Enthalpy Of Formation N2O4 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Except where otherwise noted, data are given. Standard Enthalpy Of Formation N2O4 Gas.
From www.numerade.com
SOLVED Calculate the Gibbs free energy for the formation of N2O4 gas Standard Enthalpy Of Formation N2O4 Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). The standard enthalpy of formation is a measure of the energy released or consumed when one mole. Standard Enthalpy Of Formation N2O4 Gas.
From www.numerade.com
Using Standard Enthalpy of Formation (∆H°f), calculate ∆H°RXN of Standard Enthalpy Of Formation N2O4 Gas Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition. For. Standard Enthalpy Of Formation N2O4 Gas.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation N2O4 Gas Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in. Standard Enthalpy Of Formation N2O4 Gas.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation N2O4 Gas Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Standard Enthalpy Of Formation N2O4 Gas.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation N2O4 Gas Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Except where otherwise noted, data are given for materials in their standard state (at 25. Standard Enthalpy Of Formation N2O4 Gas.
From www.chegg.com
Solved The chemistry of nitrogen oxides is very versatile. Standard Enthalpy Of Formation N2O4 Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.. Standard Enthalpy Of Formation N2O4 Gas.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation N2O4 Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Enthalpy Of Formation N2O4 Gas.
From www.chegg.com
Solved The standard heat of formation for N2O4(l) is −20.0 Standard Enthalpy Of Formation N2O4 Gas For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Except where otherwise. Standard Enthalpy Of Formation N2O4 Gas.
From www.slideserve.com
PPT Entropy PowerPoint Presentation, free download ID4499851 Standard Enthalpy Of Formation N2O4 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa).. Standard Enthalpy Of Formation N2O4 Gas.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation N2O4 Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Enthalpy Of Formation N2O4 Gas.
From www.toppr.com
The standard heat of formation of NO2(g) and N2O4(g) are 8.0 and 4.0 Standard Enthalpy Of Formation N2O4 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is. Standard Enthalpy Of Formation N2O4 Gas.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation N2O4 Gas The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Standard Enthalpy Of Formation N2O4 Gas.
From www.toppr.com
The standard heat of formation of NO2(g) and N2O4(g) are 8.0 and 4.0 Standard Enthalpy Of Formation N2O4 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). 193 rows in. Standard Enthalpy Of Formation N2O4 Gas.
From www.meritnation.com
The standard heat of formation of a no2 (g) and n2o4(g) are 8 and 4 Standard Enthalpy Of Formation N2O4 Gas Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although. Standard Enthalpy Of Formation N2O4 Gas.
From www.chegg.com
Solved Calculate ΔH∘rxn for this reaction using Standard Enthalpy Of Formation N2O4 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element in its standard state is zero by. Standard Enthalpy Of Formation N2O4 Gas.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation N2O4 Gas Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). 193 rows in chemistry and thermodynamics,. Standard Enthalpy Of Formation N2O4 Gas.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation N2O4 Gas For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the. Standard Enthalpy Of Formation N2O4 Gas.
From www.toppr.com
The standard heats of formation in kcal mol^1 of NO2(g) and N2O4(g Standard Enthalpy Of Formation N2O4 Gas For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Except where otherwise noted, data are given for materials in. Standard Enthalpy Of Formation N2O4 Gas.
From www.numerade.com
SOLVED Consider the following reaction 2NO2 (g) ===== N2O4 (g) The Standard Enthalpy Of Formation N2O4 Gas The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Except where otherwise noted, data are given for materials in. Standard Enthalpy Of Formation N2O4 Gas.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation N2O4 Gas Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table) these tables include heat. Standard Enthalpy Of Formation N2O4 Gas.
From www.toppr.com
The standard enthalpies of formation of NO2(g) and N2O4(g) 8 and 4 kcal Standard Enthalpy Of Formation N2O4 Gas For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. Except where otherwise noted, data are given. Standard Enthalpy Of Formation N2O4 Gas.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Standard Enthalpy Of Formation N2O4 Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.. Standard Enthalpy Of Formation N2O4 Gas.
From www.toppr.com
The standard enthalpies of formation of NO2(g) and N2O4(g) 8 and 4 kcal Standard Enthalpy Of Formation N2O4 Gas The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Standard Enthalpy Of Formation N2O4 Gas.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas Enthalpy Of Formation Standard Enthalpy Of Formation N2O4 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa).. Standard Enthalpy Of Formation N2O4 Gas.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation N2O4 Gas The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. Standard Enthalpy Of Formation N2O4 Gas.
From www.chegg.com
Solved Calculate the standard heat of reaction for N2O4=2NO2 Standard Enthalpy Of Formation N2O4 Gas The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation of any element in its standard state is zero by definition. Except where otherwise noted, data are given. Standard Enthalpy Of Formation N2O4 Gas.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation N2O4 Gas For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy. Standard Enthalpy Of Formation N2O4 Gas.
From byjus.com
66 Enthalpies of formation of co(g),CO2(g),N2O(g)and N2O4(g) are 110 Standard Enthalpy Of Formation N2O4 Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Except where otherwise noted, data are given for materials in their standard state. Standard Enthalpy Of Formation N2O4 Gas.
From www.numerade.com
The standard enthalpy of formation of NO2(g) and N2O4(g) are +33.18 kJ Standard Enthalpy Of Formation N2O4 Gas For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Except where otherwise noted, data are given. Standard Enthalpy Of Formation N2O4 Gas.
From www.chegg.com
Solved Table 1. Standard state enthalpies of formation, ΔH Standard Enthalpy Of Formation N2O4 Gas Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation of any element in its standard state is zero by definition. Except where otherwise noted, data are given for materials in. Standard Enthalpy Of Formation N2O4 Gas.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation N2O4 Gas 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Except where otherwise noted, data are given for materials in their standard state (at 25 °c [77 °f], 100 kpa). The standard enthalpy of formation of any element in its standard state is zero by definition. For. Standard Enthalpy Of Formation N2O4 Gas.