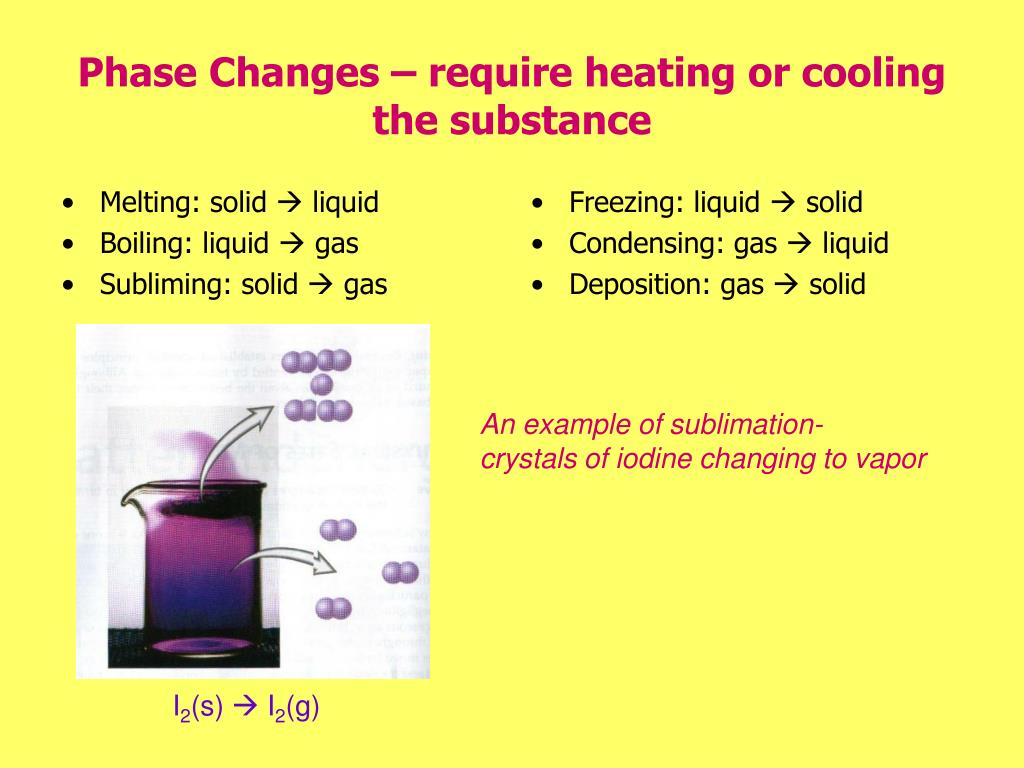
Does Deposition Require Energy . in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid state. the enthalpy of sublimation, δ hsub, is the energy required to convert one mole of a substance from the solid to the gaseous state. For example, the sublimation of carbon. Deposition is the process by which molecules. The energy change required to vaporize 1 mol of a. Deposition is when a substance in gas form changes states to become a. Sublimation is the change of state from a solid to a gas, without passing. sublimation is the process by which molecules go directly from solid into the vapor or gas phase. the energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). transitions going up (increased energy) are endothermic and require energy ,while those going down (decreased energy) are. As stated earlier, vapour deposition is the opposite of sublimation. there is an energy change associated with any phase change.
from www.slideserve.com
the energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The energy change required to vaporize 1 mol of a. For example, the sublimation of carbon. there is an energy change associated with any phase change. Deposition is when a substance in gas form changes states to become a. in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid state. sublimation is the process by which molecules go directly from solid into the vapor or gas phase. Deposition is the process by which molecules. the enthalpy of sublimation, δ hsub, is the energy required to convert one mole of a substance from the solid to the gaseous state. Sublimation is the change of state from a solid to a gas, without passing.
PPT Matter & Energy PowerPoint Presentation, free download ID5368269
Does Deposition Require Energy transitions going up (increased energy) are endothermic and require energy ,while those going down (decreased energy) are. Sublimation is the change of state from a solid to a gas, without passing. there is an energy change associated with any phase change. For example, the sublimation of carbon. transitions going up (increased energy) are endothermic and require energy ,while those going down (decreased energy) are. the energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid state. Deposition is the process by which molecules. sublimation is the process by which molecules go directly from solid into the vapor or gas phase. Deposition is when a substance in gas form changes states to become a. The energy change required to vaporize 1 mol of a. As stated earlier, vapour deposition is the opposite of sublimation. the enthalpy of sublimation, δ hsub, is the energy required to convert one mole of a substance from the solid to the gaseous state.
From slideplayer.com
Chapter 2 Matter and Change Section 2.1 Properties of Matter. ppt Does Deposition Require Energy there is an energy change associated with any phase change. the energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). transitions going up (increased energy) are endothermic and require energy ,while those going down (decreased energy) are. Deposition is when a substance in gas form changes states to become a.. Does Deposition Require Energy.
From www.researchgate.net
Working principles of (a) Directed energy deposition. Reproduced with Does Deposition Require Energy Deposition is the process by which molecules. there is an energy change associated with any phase change. in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid state. the energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). . Does Deposition Require Energy.
From ar.inspiredpencil.com
Deposition Definition Science Does Deposition Require Energy For example, the sublimation of carbon. Deposition is when a substance in gas form changes states to become a. there is an energy change associated with any phase change. in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid state. transitions going up (increased energy) are. Does Deposition Require Energy.
From www.researchgate.net
Illustration of the directed energy deposition (DED) process; the Does Deposition Require Energy Deposition is when a substance in gas form changes states to become a. in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid state. Sublimation is the change of state from a solid to a gas, without passing. As stated earlier, vapour deposition is the opposite of sublimation.. Does Deposition Require Energy.
From www.researchgate.net
Process of directed energy deposition (a) the preheating and Does Deposition Require Energy the energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The energy change required to vaporize 1 mol of a. Sublimation is the change of state from a solid to a gas, without passing. transitions going up (increased energy) are endothermic and require energy ,while those going down (decreased energy) are.. Does Deposition Require Energy.
From www.ans.org
New standard available on thermal energy deposition rates ANS Does Deposition Require Energy Deposition is when a substance in gas form changes states to become a. in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid state. sublimation is the process by which molecules go directly from solid into the vapor or gas phase. transitions going up (increased energy). Does Deposition Require Energy.
From pastpapers.us
Communikation ship inside to create for ampere my, certifications Does Deposition Require Energy there is an energy change associated with any phase change. For example, the sublimation of carbon. As stated earlier, vapour deposition is the opposite of sublimation. Deposition is when a substance in gas form changes states to become a. Deposition is the process by which molecules. in chemistry, deposition refers to the process in which a gas changes. Does Deposition Require Energy.
From apollo.nvu.vsc.edu
Latent Heats sublimation and deposition Does Deposition Require Energy transitions going up (increased energy) are endothermic and require energy ,while those going down (decreased energy) are. in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid state. The energy change required to vaporize 1 mol of a. Sublimation is the change of state from a solid. Does Deposition Require Energy.
From mechguru.com
Additive Manufacturing Technologies Directed Energy Deposition mechGuru Does Deposition Require Energy sublimation is the process by which molecules go directly from solid into the vapor or gas phase. The energy change required to vaporize 1 mol of a. the energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). there is an energy change associated with any phase change. For example, the. Does Deposition Require Energy.
From www.researchgate.net
Direct energy deposition process Download Scientific Diagram Does Deposition Require Energy in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid state. Deposition is when a substance in gas form changes states to become a. transitions going up (increased energy) are endothermic and require energy ,while those going down (decreased energy) are. there is an energy change. Does Deposition Require Energy.
From additec3d.com
Introduction to Directed Energy Deposition (DED) Does Deposition Require Energy Sublimation is the change of state from a solid to a gas, without passing. The energy change required to vaporize 1 mol of a. Deposition is when a substance in gas form changes states to become a. the enthalpy of sublimation, δ hsub, is the energy required to convert one mole of a substance from the solid to the. Does Deposition Require Energy.
From estudyassistant.com
Which list includes the phase changes that require a loss of energy Does Deposition Require Energy Sublimation is the change of state from a solid to a gas, without passing. transitions going up (increased energy) are endothermic and require energy ,while those going down (decreased energy) are. there is an energy change associated with any phase change. Deposition is the process by which molecules. sublimation is the process by which molecules go directly. Does Deposition Require Energy.
From www.slideserve.com
PPT Energy and Phase Changes PowerPoint Presentation, free download Does Deposition Require Energy transitions going up (increased energy) are endothermic and require energy ,while those going down (decreased energy) are. Deposition is when a substance in gas form changes states to become a. The energy change required to vaporize 1 mol of a. As stated earlier, vapour deposition is the opposite of sublimation. For example, the sublimation of carbon. in chemistry,. Does Deposition Require Energy.
From theinnovationof.com
Understanding the Complexities of Deposition Definition Science Does Deposition Require Energy there is an energy change associated with any phase change. sublimation is the process by which molecules go directly from solid into the vapor or gas phase. The energy change required to vaporize 1 mol of a. the enthalpy of sublimation, δ hsub, is the energy required to convert one mole of a substance from the solid. Does Deposition Require Energy.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Does Deposition Require Energy For example, the sublimation of carbon. there is an energy change associated with any phase change. Deposition is the process by which molecules. the enthalpy of sublimation, δ hsub, is the energy required to convert one mole of a substance from the solid to the gaseous state. Deposition is when a substance in gas form changes states to. Does Deposition Require Energy.
From studylib.net
Stream deposition Where does stream deposition occur? At mouth Does Deposition Require Energy there is an energy change associated with any phase change. the energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Sublimation is the change of state from a solid to a gas, without passing. The energy change required to vaporize 1 mol of a. sublimation is the process by which. Does Deposition Require Energy.
From sciencenotes.org
Phase Changes of Matter (Phase Transitions) Does Deposition Require Energy the energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). sublimation is the process by which molecules go directly from solid into the vapor or gas phase. The energy change required to vaporize 1 mol of a. the enthalpy of sublimation, δ hsub, is the energy required to convert one. Does Deposition Require Energy.
From sciencestruck.com
What is Deposition? Science Struck Does Deposition Require Energy in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid state. Sublimation is the change of state from a solid to a gas, without passing. the energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). the enthalpy of sublimation,. Does Deposition Require Energy.
From slideplayer.com
Matter Properties & Changes ppt download Does Deposition Require Energy the enthalpy of sublimation, δ hsub, is the energy required to convert one mole of a substance from the solid to the gaseous state. Deposition is the process by which molecules. The energy change required to vaporize 1 mol of a. in chemistry, deposition refers to the process in which a gas changes directly to a solid without. Does Deposition Require Energy.
From lawrina.org
What is a Deposition? Legal Definition & Process Lawrina Does Deposition Require Energy in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid state. the enthalpy of sublimation, δ hsub, is the energy required to convert one mole of a substance from the solid to the gaseous state. The energy change required to vaporize 1 mol of a. Deposition is. Does Deposition Require Energy.
From www.researchgate.net
The directed energy deposition process [2]. Download Scientific Diagram Does Deposition Require Energy sublimation is the process by which molecules go directly from solid into the vapor or gas phase. For example, the sublimation of carbon. The energy change required to vaporize 1 mol of a. the energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Sublimation is the change of state from a. Does Deposition Require Energy.
From www.expii.com
Deposition — Definition & Overview Expii Does Deposition Require Energy Deposition is when a substance in gas form changes states to become a. sublimation is the process by which molecules go directly from solid into the vapor or gas phase. Deposition is the process by which molecules. in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid. Does Deposition Require Energy.
From makenica.com
A COMPLETE GUIDE DIRECT ENERGY DEPOSITION TECHNOLOGY Does Deposition Require Energy transitions going up (increased energy) are endothermic and require energy ,while those going down (decreased energy) are. Sublimation is the change of state from a solid to a gas, without passing. Deposition is when a substance in gas form changes states to become a. As stated earlier, vapour deposition is the opposite of sublimation. in chemistry, deposition refers. Does Deposition Require Energy.
From mungfali.com
6 Phases Of Matter Does Deposition Require Energy sublimation is the process by which molecules go directly from solid into the vapor or gas phase. For example, the sublimation of carbon. Deposition is the process by which molecules. Deposition is when a substance in gas form changes states to become a. there is an energy change associated with any phase change. As stated earlier, vapour deposition. Does Deposition Require Energy.
From www.revimage.org
How Does Weathering Erosion And Deposition Affect Earth S Surface The Does Deposition Require Energy sublimation is the process by which molecules go directly from solid into the vapor or gas phase. For example, the sublimation of carbon. the energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Sublimation is the change of state from a solid to a gas, without passing. in chemistry, deposition. Does Deposition Require Energy.
From www.researchgate.net
Energydeposition distributions of 0.5MeV electrons incident on Does Deposition Require Energy The energy change required to vaporize 1 mol of a. Deposition is the process by which molecules. in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid state. As stated earlier, vapour deposition is the opposite of sublimation. sublimation is the process by which molecules go directly. Does Deposition Require Energy.
From www.youtube.com
Directed Energy Deposition (DED) An Additive Manufacturing Technique Does Deposition Require Energy sublimation is the process by which molecules go directly from solid into the vapor or gas phase. the enthalpy of sublimation, δ hsub, is the energy required to convert one mole of a substance from the solid to the gaseous state. Sublimation is the change of state from a solid to a gas, without passing. transitions going. Does Deposition Require Energy.
From www.chegg.com
Solved 19 of 58 Review Which of the following changes in Does Deposition Require Energy in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid state. For example, the sublimation of carbon. the enthalpy of sublimation, δ hsub, is the energy required to convert one mole of a substance from the solid to the gaseous state. transitions going up (increased energy). Does Deposition Require Energy.
From www.mdpi.com
JMMP Free FullText Investigation of Effect of Processing Does Deposition Require Energy there is an energy change associated with any phase change. Deposition is when a substance in gas form changes states to become a. As stated earlier, vapour deposition is the opposite of sublimation. The energy change required to vaporize 1 mol of a. sublimation is the process by which molecules go directly from solid into the vapor or. Does Deposition Require Energy.
From exobrpuio.blob.core.windows.net
How Do Weathering Erosion And Deposition Interact Work Together at Does Deposition Require Energy in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid state. there is an energy change associated with any phase change. the enthalpy of sublimation, δ hsub, is the energy required to convert one mole of a substance from the solid to the gaseous state. The. Does Deposition Require Energy.
From www.researchgate.net
Schematic overview of the directed energy deposition system and Does Deposition Require Energy the enthalpy of sublimation, δ hsub, is the energy required to convert one mole of a substance from the solid to the gaseous state. Deposition is the process by which molecules. transitions going up (increased energy) are endothermic and require energy ,while those going down (decreased energy) are. The energy change required to vaporize 1 mol of a.. Does Deposition Require Energy.
From stock.adobe.com
Physical states of matter.Solid, liquid and gas.Melting, freezing Does Deposition Require Energy the enthalpy of sublimation, δ hsub, is the energy required to convert one mole of a substance from the solid to the gaseous state. there is an energy change associated with any phase change. As stated earlier, vapour deposition is the opposite of sublimation. Deposition is when a substance in gas form changes states to become a. . Does Deposition Require Energy.
From ampower.eu
Direct Energy Deposition AMPOWER Does Deposition Require Energy the enthalpy of sublimation, δ hsub, is the energy required to convert one mole of a substance from the solid to the gaseous state. Deposition is the process by which molecules. Deposition is when a substance in gas form changes states to become a. sublimation is the process by which molecules go directly from solid into the vapor. Does Deposition Require Energy.
From www.researchgate.net
Energy deposition at the focus. Download Scientific Diagram Does Deposition Require Energy The energy change required to vaporize 1 mol of a. Deposition is when a substance in gas form changes states to become a. Sublimation is the change of state from a solid to a gas, without passing. Deposition is the process by which molecules. in chemistry, deposition refers to the process in which a gas changes directly to a. Does Deposition Require Energy.
From www.slideserve.com
PPT Matter & Energy PowerPoint Presentation, free download ID5368269 Does Deposition Require Energy transitions going up (increased energy) are endothermic and require energy ,while those going down (decreased energy) are. in chemistry, deposition refers to the process in which a gas changes directly to a solid without going through the liquid state. As stated earlier, vapour deposition is the opposite of sublimation. the enthalpy of sublimation, δ hsub, is the. Does Deposition Require Energy.