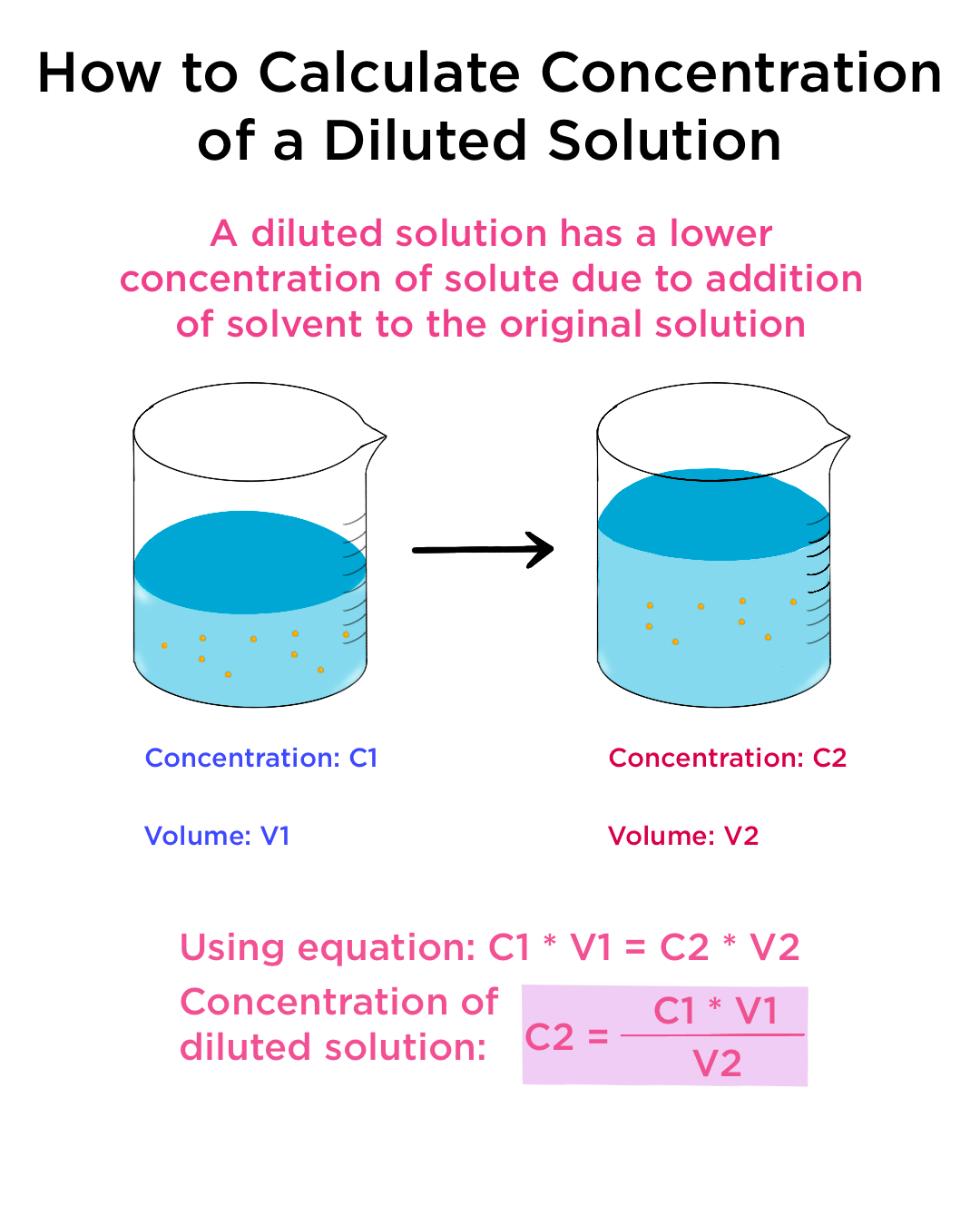
Dilution Acid Definition . It doesn’t make the acid weaker or less reactive. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you are working in. Dilution of an acid or base means mixing an acid or base with water. State whether the concentration of a solution is directly or indirectly proportional to its volume. All acids may be an organic or. What is called dilution of acid? Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilute acids contain a large amount of water. This is done to decrease the concentration of. A concentrated acid can be diluted with the addition of water. You dilute acids to lower the amount of dissolved substance in the solution. Concentration is the removal of solvent, which increases the.
from www.expii.com
Concentration is the removal of solvent, which increases the. Dilute acids contain a large amount of water. State whether the concentration of a solution is directly or indirectly proportional to its volume. What is called dilution of acid? A concentrated acid can be diluted with the addition of water. All acids may be an organic or. Dilution of an acid or base means mixing an acid or base with water. You dilute acids to lower the amount of dissolved substance in the solution. It doesn’t make the acid weaker or less reactive. This is done to decrease the concentration of.
Dilution of Solutions — Overview & Examples Expii
Dilution Acid Definition It doesn’t make the acid weaker or less reactive. Concentration is the removal of solvent, which increases the. You dilute acids to lower the amount of dissolved substance in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you are working in. A concentrated acid can be diluted with the addition of water. Dilute acids contain a large amount of water. State whether the concentration of a solution is directly or indirectly proportional to its volume. All acids may be an organic or. It doesn’t make the acid weaker or less reactive. This is done to decrease the concentration of. What is called dilution of acid? Dilution of an acid or base means mixing an acid or base with water.
From ar.inspiredpencil.com
Dilute Acid Dilution Acid Definition It doesn’t make the acid weaker or less reactive. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases the. All acids may be an organic or. Dilution of an acid or base means mixing an acid or base with water. Dilution is the addition of solvent,. Dilution Acid Definition.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Acid Definition You dilute acids to lower the amount of dissolved substance in the solution. This is done to decrease the concentration of. What is called dilution of acid? A concentrated acid can be diluted with the addition of water. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which. Dilution Acid Definition.
From chemnotcheem.com
Definition and properties of acids O Level Chemistry Notes Dilution Acid Definition All acids may be an organic or. It doesn’t make the acid weaker or less reactive. Dilute acids contain a large amount of water. State whether the concentration of a solution is directly or indirectly proportional to its volume. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory. Dilution Acid Definition.
From www.slideshare.net
Acids And Bases Dilution Acid Definition Concentration is the removal of solvent, which increases the. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution of an acid or base means mixing an acid or base with water. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you. Dilution Acid Definition.
From www.slideserve.com
PPT The effect of dilution on the pH of an acid PowerPoint Dilution Acid Definition The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you are working in. This is done to decrease the concentration of. All acids may be an organic or. You dilute acids to lower the amount of dissolved substance in the solution. Dilution is the addition of solvent, which. Dilution Acid Definition.
From study.com
How to Dilute a Strong Acid Solution to a Given pH Chemistry Dilution Acid Definition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. You dilute acids to lower the amount of dissolved substance in the solution. Dilution of an acid or base means mixing an acid or base with water. Concentration is the removal of solvent, which increases the. It doesn’t make the acid weaker or less. Dilution Acid Definition.
From www.youtube.com
Concentrated and Dilute Acids Acid, Bases and Salts 7th Class Dilution Acid Definition All acids may be an organic or. Concentration is the removal of solvent, which increases the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. It doesn’t make the acid weaker or less reactive. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of. Dilution Acid Definition.
From wordwall.net
Dilution of Acids and Alkalis Find the match Dilution Acid Definition The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you are working in. What is called dilution of acid? Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. It doesn’t make the acid weaker or less reactive. All acids may be. Dilution Acid Definition.
From ablogwithadifference.com
Weak Acid and Dilute Acid The best 7 difference Dilution Acid Definition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. This is done to decrease the concentration of. You dilute acids to lower the amount of dissolved substance in the solution. A concentrated acid can be diluted with the addition of water. It doesn’t make the acid weaker or less reactive. Concentration is the. Dilution Acid Definition.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Acid Definition State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. You dilute acids to lower the amount of dissolved substance in the solution. The desired concentration and volume of acid is typically determined by a school assignment or by. Dilution Acid Definition.
From www.w3schools.blog
Standard enthalpy of dilution W3schools Dilution Acid Definition A concentrated acid can be diluted with the addition of water. State whether the concentration of a solution is directly or indirectly proportional to its volume. You dilute acids to lower the amount of dissolved substance in the solution. Dilute acids contain a large amount of water. It doesn’t make the acid weaker or less reactive. What is called dilution. Dilution Acid Definition.
From testbook.com
Dilute Acid Learn Definition, Examples, Formula and Properties Dilution Acid Definition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. You dilute acids to lower the amount of dissolved substance in the solution. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you are working in. All acids may be an organic. Dilution Acid Definition.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Acid Definition A concentrated acid can be diluted with the addition of water. It doesn’t make the acid weaker or less reactive. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. This is done to decrease the concentration of. You dilute acids to lower the amount of dissolved substance in the solution. All acids may. Dilution Acid Definition.
From www.youtube.com
Dilution of AcidWhy is it to add acid to water but not Dilution Acid Definition Dilution of an acid or base means mixing an acid or base with water. A concentrated acid can be diluted with the addition of water. It doesn’t make the acid weaker or less reactive. Dilute acids contain a large amount of water. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. This is. Dilution Acid Definition.
From www.wikihow.com
ImageDilute an Acid Step 16.jpg wikiHow Dilution Acid Definition A concentrated acid can be diluted with the addition of water. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you are working in. Dilute acids contain a large amount of water. It doesn’t make the acid weaker or less reactive. Dilution is the addition of solvent, which. Dilution Acid Definition.
From sciencenotes.org
Add Acid to Water or Water to Acid? Safely Diluting Acids Dilution Acid Definition Dilute acids contain a large amount of water. You dilute acids to lower the amount of dissolved substance in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A concentrated acid can be diluted with the addition of water. Concentration is the removal of solvent, which increases the. It doesn’t make. Dilution Acid Definition.
From www.youtube.com
Concentrated/dilute acid Dilution of acid Basicity of acid 10th Dilution Acid Definition State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution of an acid or base means mixing an acid or base with water. Concentration is the removal of solvent, which increases the. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you. Dilution Acid Definition.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of Dilution Acid Definition Dilute acids contain a large amount of water. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you are working in. You dilute acids to lower the amount of dissolved substance in. Dilution Acid Definition.
From www.slideserve.com
PPT Biology practical ( Food Tests) PowerPoint Presentation, free Dilution Acid Definition A concentrated acid can be diluted with the addition of water. This is done to decrease the concentration of. You dilute acids to lower the amount of dissolved substance in the solution. Dilution of an acid or base means mixing an acid or base with water. State whether the concentration of a solution is directly or indirectly proportional to its. Dilution Acid Definition.
From www.youtube.com
DIlution of acids and bases YouTube Dilution Acid Definition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilute acids contain a large amount of water. A concentrated acid can be diluted with the addition of water. What. Dilution Acid Definition.
From www.slideserve.com
PPT The effect of dilution on the pH of an acid PowerPoint Dilution Acid Definition Dilute acids contain a large amount of water. All acids may be an organic or. A concentrated acid can be diluted with the addition of water. What is called dilution of acid? This is done to decrease the concentration of. You dilute acids to lower the amount of dissolved substance in the solution. Concentration is the removal of solvent, which. Dilution Acid Definition.
From www.studypool.com
SOLUTION Dilution chemistry Studypool Dilution Acid Definition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. This is done to decrease the concentration of. Dilute acids contain a large amount of water. It doesn’t make the acid weaker or less reactive. A concentrated acid can be diluted with the addition of water. State whether the concentration of a solution is. Dilution Acid Definition.
From studylib.net
Lesson 4 Diluting Acids and Alkalis Dilution Acid Definition All acids may be an organic or. It doesn’t make the acid weaker or less reactive. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. A concentrated acid can be diluted with the addition of water. Dilute acids. Dilution Acid Definition.
From www.youtube.com
CBSE Class 10 Chemistry Notes Dilution YouTube Dilution Acid Definition The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you are working in. What is called dilution of acid? State whether the concentration of a solution is directly or indirectly proportional to its volume. All acids may be an organic or. Dilution of an acid or base means. Dilution Acid Definition.
From www.youtube.com
3. Strong, weak, dilute and concentrated acids (HSC chemistry) YouTube Dilution Acid Definition Dilute acids contain a large amount of water. This is done to decrease the concentration of. A concentrated acid can be diluted with the addition of water. State whether the concentration of a solution is directly or indirectly proportional to its volume. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements. Dilution Acid Definition.
From ar.inspiredpencil.com
Dilute Acid Dilution Acid Definition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you are working in. What is called dilution of acid? Dilution of an acid or base means mixing an acid or base with. Dilution Acid Definition.
From www.pinterest.com
Dilute Flashcards, Easy science, Science student Dilution Acid Definition Concentration is the removal of solvent, which increases the. You dilute acids to lower the amount of dissolved substance in the solution. A concentrated acid can be diluted with the addition of water. This is done to decrease the concentration of. All acids may be an organic or. State whether the concentration of a solution is directly or indirectly proportional. Dilution Acid Definition.
From ar.inspiredpencil.com
Dilute Acid Dilution Acid Definition A concentrated acid can be diluted with the addition of water. Dilution of an acid or base means mixing an acid or base with water. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. It doesn’t make the acid weaker or less reactive. You dilute acids to lower the amount of dissolved substance. Dilution Acid Definition.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Acid Definition All acids may be an organic or. It doesn’t make the acid weaker or less reactive. Concentration is the removal of solvent, which increases the. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The desired concentration and. Dilution Acid Definition.
From www.youtube.com
Acids, Bases and Salts Class X Science Definition, Classification Dilution Acid Definition You dilute acids to lower the amount of dissolved substance in the solution. Concentration is the removal of solvent, which increases the. It doesn’t make the acid weaker or less reactive. Dilute acids contain a large amount of water. Dilution of an acid or base means mixing an acid or base with water. A concentrated acid can be diluted with. Dilution Acid Definition.
From www.sliderbase.com
Strengths of Acids and Bases Making Dilutions Presentation Chemistry Dilution Acid Definition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you are working in. It doesn’t make the acid weaker or less reactive. This is done to decrease the concentration of. A concentrated. Dilution Acid Definition.
From www.slideserve.com
PPT Qualitative Analysis Carry out procedures to identify ions in Dilution Acid Definition Concentration is the removal of solvent, which increases the. State whether the concentration of a solution is directly or indirectly proportional to its volume. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you are working in. You dilute acids to lower the amount of dissolved substance in. Dilution Acid Definition.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Acid Definition Dilution of an acid or base means mixing an acid or base with water. What is called dilution of acid? Concentration is the removal of solvent, which increases the. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you are working in. You dilute acids to lower the. Dilution Acid Definition.
From www.slideshare.net
Chapter 16 solutions Dilution Acid Definition Concentration is the removal of solvent, which increases the. Dilution of an acid or base means mixing an acid or base with water. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you are working in. State whether the concentration of a solution is directly or indirectly proportional. Dilution Acid Definition.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Acid Definition What is called dilution of acid? You dilute acids to lower the amount of dissolved substance in the solution. All acids may be an organic or. A concentrated acid can be diluted with the addition of water. State whether the concentration of a solution is directly or indirectly proportional to its volume. This is done to decrease the concentration of.. Dilution Acid Definition.