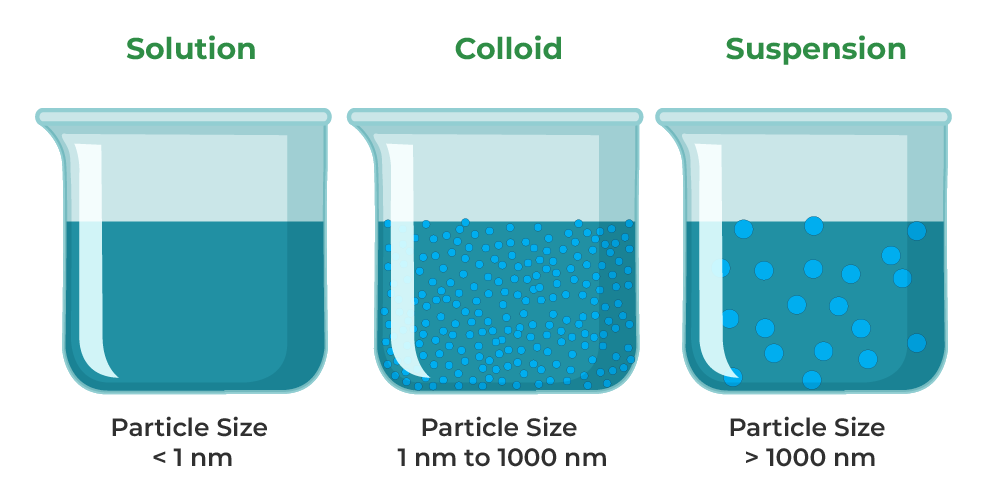
Suspension Colloid True . Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. In the mixture of salt in water, sand in water and milk, salt in water is a true solution, sand in water is the suspension,. A true solution is a homogeneous mixture of two or more. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a.
from www.geeksforgeeks.org
A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. In the mixture of salt in water, sand in water and milk, salt in water is a true solution, sand in water is the suspension,. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. A true solution is a homogeneous mixture of two or more. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform.
Suspension(Chemistry) Definition, Properties, Examples, and FAQs
Suspension Colloid True Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. A true solution is a homogeneous mixture of two or more. In the mixture of salt in water, sand in water and milk, salt in water is a true solution, sand in water is the suspension,. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and.
From 88guru.com
True solution, Suspension, Colloidal Solutions 88Guru Suspension Colloid True In the mixture of salt in water, sand in water and milk, salt in water is a true solution, sand in water is the suspension,. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while. Suspension Colloid True.
From brainly.in
How are colloids and suspensions different from solutions ? Brainly.in Suspension Colloid True A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. A true solution is a homogeneous mixture of two or more. In the mixture of salt in water, sand in. Suspension Colloid True.
From www.geeksforgeeks.org
Colloids Definition, Classification, Application, Properties, & FAQs Suspension Colloid True Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. A true solution is a homogeneous mixture of two or more. In the mixture of salt in water, sand in water and milk, salt in water is a true solution, sand in water is the suspension,.. Suspension Colloid True.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Suspension Colloid True In the mixture of salt in water, sand in water and milk, salt in water is a true solution, sand in water is the suspension,. A true solution is a homogeneous mixture of two or more. Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. A colloid is a heterogeneous. Suspension Colloid True.
From ar.inspiredpencil.com
Colloid Suspension Solution Suspension Colloid True Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. A true solution is a homogeneous mixture of two or more. Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. In the mixture of salt in water, sand in water and milk,. Suspension Colloid True.
From www.numerade.com
SOLVED The images depict solid particles of different diameters in a Suspension Colloid True In the mixture of salt in water, sand in water and milk, salt in water is a true solution, sand in water is the suspension,. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. A true solution is a homogeneous mixture of two or more.. Suspension Colloid True.
From ar.inspiredpencil.com
Colloids Suspensions And Solutions Suspension Colloid True A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. In the mixture of salt in water, sand in water and milk, salt in water is a. Suspension Colloid True.
From edurev.in
Difference between true solution, suspension and colloidal sol Suspension Colloid True Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. In the mixture of salt in water, sand in water and milk, salt in water is a true solution, sand in water is the suspension,. A colloid is a type of mixture where small particles of one substance are suspended and. Suspension Colloid True.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID2182578 Suspension Colloid True Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A true. Suspension Colloid True.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Suspension Colloid True A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. In the mixture of salt in water, sand in water and milk, salt in water is a true solution, sand in water is the suspension,. Suspensions and colloids are two common types of mixtures whose properties are in many. Suspension Colloid True.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID2182578 Suspension Colloid True Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Suspensions and colloids are. Suspension Colloid True.
From mungfali.com
10 Examples Of Colloids Suspension Colloid True Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. A true solution is a homogeneous mixture of two or more. A colloid is a type of mixture. Suspension Colloid True.
From plantlet.org
Mixture Types Solution, Suspension, Colloids & Others Plantlet Suspension Colloid True A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. A true solution is a homogeneous mixture of two or more. A colloid is a heterogeneous mixture in which the dispersed. Suspension Colloid True.
From brainly.in
draw diagram illustrate true solutions suspension and colloidal Suspension Colloid True In the mixture of salt in water, sand in water and milk, salt in water is a true solution, sand in water is the suspension,. A true solution is a homogeneous mixture of two or more. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. Colloidal and suspension solutions have visible. Suspension Colloid True.
From www.slideserve.com
PPT Matter Properties & Change PowerPoint Presentation, free Suspension Colloid True In the mixture of salt in water, sand in water and milk, salt in water is a true solution, sand in water is the suspension,. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. A colloid is a heterogeneous mixture in which the dispersed particles. Suspension Colloid True.
From pt.slideshare.net
Solutions, suspensions, and colloids Suspension Colloid True A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. In the. Suspension Colloid True.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Suspension Colloid True Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. A true solution is a homogeneous mixture of two or more. Suspensions and colloids are two common types of mixtures whose. Suspension Colloid True.
From www.youtube.com
Class 9 / true solution suspension colloid / and properties with Suspension Colloid True Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. A colloid. Suspension Colloid True.
From www.youtube.com
SCIENCE 6 SUSPENSION AND COLLOID YouTube Suspension Colloid True A true solution is a homogeneous mixture of two or more. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. In the mixture of. Suspension Colloid True.
From www.youtube.com
Difference Between a True Solution, Suspension and Colloid Chemistry Suspension Colloid True Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. In the mixture of salt in water, sand in water and milk, salt in water is a true solution, sand in water is the suspension,. A colloid is a type of mixture where small particles of one substance are suspended and. Suspension Colloid True.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Colloid True A true solution is a homogeneous mixture of two or more. Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A colloid is a type of mixture where small particles of. Suspension Colloid True.
From www.youtube.com
Science Quiz Solution, Suspension or Colloid ANY 10 YouTube Suspension Colloid True Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A true solution is a homogeneous mixture of two or more. A colloid is a type of. Suspension Colloid True.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Suspension Colloid True A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A. Suspension Colloid True.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Suspension Colloid True Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A. Suspension Colloid True.
From www.sliderbase.com
Suspensions and Colloids Presentation Chemistry Suspension Colloid True Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution. Suspension Colloid True.
From scsscience9.blogspot.com
Is Matter Around Us Pure? Blog 6 Suspension Colloid True A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. Suspensions and. Suspension Colloid True.
From byjus.com
Suspensions (Chemistry) Definition, Properties, Examples with Videos Suspension Colloid True A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. In the. Suspension Colloid True.
From www.youtube.com
SCATTERING OF LIGHT BY TRUE SOLUTION, SUSPENSION AND COLLOIDS (class IX Suspension Colloid True A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A true solution is a homogeneous mixture of two or more. Colloidal and suspension solutions have visible particles that stay. Suspension Colloid True.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Suspension Colloid True A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true. Suspension Colloid True.
From www.youtube.com
Solution Suspension Colloid YouTube Suspension Colloid True Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. A true solution is a homogeneous mixture of two or more. A colloid is a heterogeneous mixture in which the. Suspension Colloid True.
From www.slideserve.com
PPT SOLUTION CHEMISTRY PowerPoint Presentation, free download ID Suspension Colloid True Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. In the mixture of salt in water, sand in water and milk, salt in water is a true solution, sand in water is the suspension,. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between. Suspension Colloid True.
From www.thoughtco.com
Tyndall Effect Definition and Examples Suspension Colloid True Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears. Suspension Colloid True.
From www.slideshare.net
Colloids Suspension Colloid True Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. A true solution is a homogeneous mixture of two or more. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. Colloidal and suspension solutions have visible particles. Suspension Colloid True.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Suspension Colloid True Colloidal and suspension solutions have visible particles that stay suspended in the solvent, while a true solution appears uniform. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and. A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. A colloid. Suspension Colloid True.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Colloid True A colloid is a type of mixture where small particles of one substance are suspended and distributed throughout a continuous medium. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A true solution is a homogeneous mixture of two or more. Colloidal solution is a heterogeneous mixture in which particle. Suspension Colloid True.