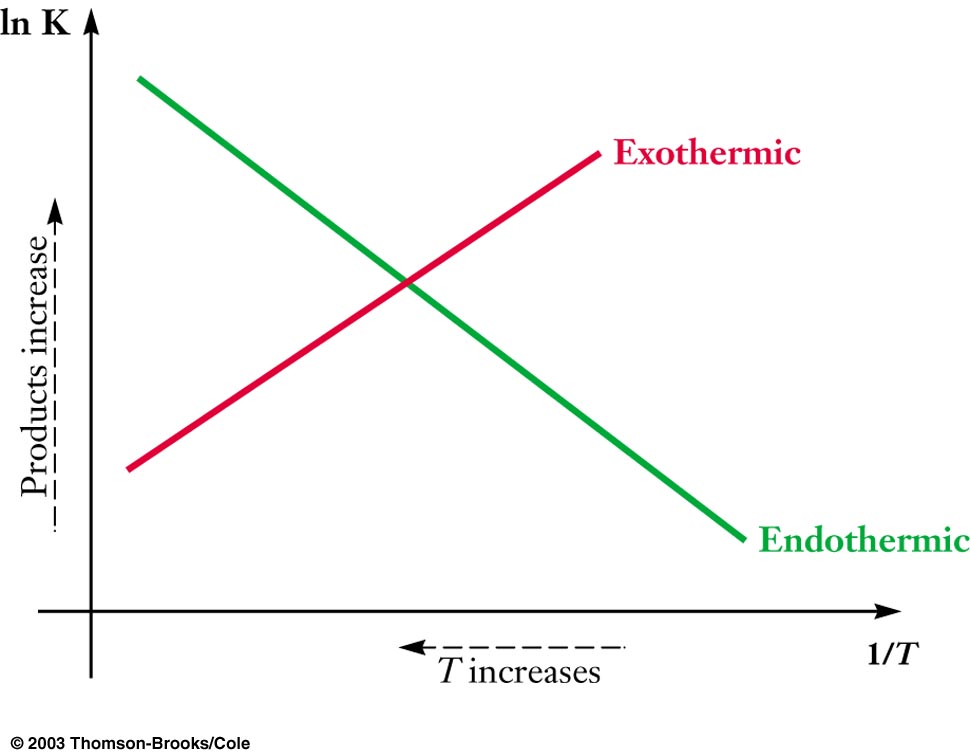
Endothermic Reaction And Entropy . Predict whether entropy change for a reaction is increasing or. Recall the meaning of exothermic and endothermic. A spontaneous endothermic reaction occurs when the enthalpy changes. When ice melts, the change is endothermic (h is positive), and entropy increases (s is positive) as the water molecules lose ordered arrangement. This condition describes an endothermic process that involves a decrease in system entropy. Some endothermic reactions are able to occur spontaneously at room temperature. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. Bond energies (the amount of energy that must be added in order to break a bond) are an. Since entropy increases when temperature increases, the entropy of surroundings increases during an exothermic. This shows how enthalpy is not the only driver of chemical. In this case, δ g will be positive regardless of the temperature. The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly freezes into slush.
from socratic.org
The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly freezes into slush. This shows how enthalpy is not the only driver of chemical. In this case, δ g will be positive regardless of the temperature. Bond energies (the amount of energy that must be added in order to break a bond) are an. Some endothermic reactions are able to occur spontaneously at room temperature. This condition describes an endothermic process that involves a decrease in system entropy. A spontaneous endothermic reaction occurs when the enthalpy changes. When ice melts, the change is endothermic (h is positive), and entropy increases (s is positive) as the water molecules lose ordered arrangement. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. Recall the meaning of exothermic and endothermic.
How do I relate equilibrium constants to temperature change to find the
Endothermic Reaction And Entropy This shows how enthalpy is not the only driver of chemical. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. This shows how enthalpy is not the only driver of chemical. In this case, δ g will be positive regardless of the temperature. Bond energies (the amount of energy that must be added in order to break a bond) are an. The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly freezes into slush. A spontaneous endothermic reaction occurs when the enthalpy changes. Since entropy increases when temperature increases, the entropy of surroundings increases during an exothermic. Some endothermic reactions are able to occur spontaneously at room temperature. This condition describes an endothermic process that involves a decrease in system entropy. When ice melts, the change is endothermic (h is positive), and entropy increases (s is positive) as the water molecules lose ordered arrangement. Predict whether entropy change for a reaction is increasing or. Recall the meaning of exothermic and endothermic.
From techschematic.com
The Enthalpy Diagram of an Endothermic Reaction Explained Endothermic Reaction And Entropy Recall the meaning of exothermic and endothermic. This condition describes an endothermic process that involves a decrease in system entropy. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. This shows how enthalpy is not the only driver of chemical. In this case, δ g will be positive regardless of the temperature.. Endothermic Reaction And Entropy.
From byjus.com
which of the following reaction is said to be entropy driven 1 Endothermic Reaction And Entropy Predict whether entropy change for a reaction is increasing or. Some endothermic reactions are able to occur spontaneously at room temperature. This condition describes an endothermic process that involves a decrease in system entropy. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. In this case, δ g will be positive regardless. Endothermic Reaction And Entropy.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction And Entropy Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. Some endothermic reactions are able to occur spontaneously at room temperature. Since entropy increases when temperature increases, the entropy of surroundings increases during an exothermic. A spontaneous endothermic reaction occurs when the enthalpy changes. This condition describes an endothermic process that involves a. Endothermic Reaction And Entropy.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction And Entropy Bond energies (the amount of energy that must be added in order to break a bond) are an. Recall the meaning of exothermic and endothermic. When ice melts, the change is endothermic (h is positive), and entropy increases (s is positive) as the water molecules lose ordered arrangement. A spontaneous endothermic reaction occurs when the enthalpy changes. Since entropy increases. Endothermic Reaction And Entropy.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Endothermic Reaction And Entropy In this case, δ g will be positive regardless of the temperature. A spontaneous endothermic reaction occurs when the enthalpy changes. Some endothermic reactions are able to occur spontaneously at room temperature. Since entropy increases when temperature increases, the entropy of surroundings increases during an exothermic. Bond energies (the amount of energy that must be added in order to break. Endothermic Reaction And Entropy.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction And Entropy A spontaneous endothermic reaction occurs when the enthalpy changes. Some endothermic reactions are able to occur spontaneously at room temperature. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. Recall the meaning of exothermic and endothermic. Predict whether entropy change for a reaction is increasing or. Bond energies (the amount of energy. Endothermic Reaction And Entropy.
From resolutionsforyou.com
The Journey of an Endothermic Reaction Understanding the Reaction Endothermic Reaction And Entropy A spontaneous endothermic reaction occurs when the enthalpy changes. Since entropy increases when temperature increases, the entropy of surroundings increases during an exothermic. This shows how enthalpy is not the only driver of chemical. This condition describes an endothermic process that involves a decrease in system entropy. When ice melts, the change is endothermic (h is positive), and entropy increases. Endothermic Reaction And Entropy.
From slideplayer.com
Endothermic Vs. Exothermic Reaction Graphs ppt download Endothermic Reaction And Entropy Some endothermic reactions are able to occur spontaneously at room temperature. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. Predict whether entropy change for a reaction is increasing or. The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly freezes. Endothermic Reaction And Entropy.
From www.slideserve.com
PPT Entropy PowerPoint Presentation, free download ID2799024 Endothermic Reaction And Entropy This condition describes an endothermic process that involves a decrease in system entropy. Bond energies (the amount of energy that must be added in order to break a bond) are an. Some endothermic reactions are able to occur spontaneously at room temperature. When ice melts, the change is endothermic (h is positive), and entropy increases (s is positive) as the. Endothermic Reaction And Entropy.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Endothermic Reaction And Entropy A spontaneous endothermic reaction occurs when the enthalpy changes. This shows how enthalpy is not the only driver of chemical. This condition describes an endothermic process that involves a decrease in system entropy. The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly freezes into slush. Recall the meaning. Endothermic Reaction And Entropy.
From www.numerade.com
SOLVEDHow does the entropy of the surroundings change during an Endothermic Reaction And Entropy Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. This condition describes an endothermic process that involves a decrease in system entropy. A spontaneous endothermic reaction occurs when the enthalpy changes. This shows how enthalpy is not the only driver of chemical. Bond energies (the amount of energy that must be added. Endothermic Reaction And Entropy.
From general.chemistrysteps.com
What is Enthalpy Chemistry Steps Endothermic Reaction And Entropy In this case, δ g will be positive regardless of the temperature. This condition describes an endothermic process that involves a decrease in system entropy. A spontaneous endothermic reaction occurs when the enthalpy changes. Since entropy increases when temperature increases, the entropy of surroundings increases during an exothermic. Recall the meaning of exothermic and endothermic. The reaction of barium hydroxide. Endothermic Reaction And Entropy.
From general.chemistrysteps.com
Entropy Changes in the Surroundings Chemistry Steps Endothermic Reaction And Entropy This condition describes an endothermic process that involves a decrease in system entropy. When ice melts, the change is endothermic (h is positive), and entropy increases (s is positive) as the water molecules lose ordered arrangement. Recall the meaning of exothermic and endothermic. This shows how enthalpy is not the only driver of chemical. Some endothermic reactions are able to. Endothermic Reaction And Entropy.
From improove.in
Experiment 16 Heat generation during reaction Improove Endothermic Reaction And Entropy Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly freezes into slush. In this case, δ g will be positive regardless of the temperature. This shows how enthalpy is not the. Endothermic Reaction And Entropy.
From www.youtube.com
Thermodynamics Exo and Endothermic Reactions YouTube Endothermic Reaction And Entropy Bond energies (the amount of energy that must be added in order to break a bond) are an. A spontaneous endothermic reaction occurs when the enthalpy changes. Predict whether entropy change for a reaction is increasing or. The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly freezes into. Endothermic Reaction And Entropy.
From www.youtube.com
Endothermic and exothermic reactions. Enthalpy YouTube Endothermic Reaction And Entropy Bond energies (the amount of energy that must be added in order to break a bond) are an. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. Since entropy increases when temperature increases, the entropy of surroundings increases during an exothermic. The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but. Endothermic Reaction And Entropy.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction And Entropy Bond energies (the amount of energy that must be added in order to break a bond) are an. This shows how enthalpy is not the only driver of chemical. In this case, δ g will be positive regardless of the temperature. This condition describes an endothermic process that involves a decrease in system entropy. Since entropy increases when temperature increases,. Endothermic Reaction And Entropy.
From www.doubtnut.com
For an endothermic reaction energy of activation is E(a) and enthlpy o Endothermic Reaction And Entropy This condition describes an endothermic process that involves a decrease in system entropy. When ice melts, the change is endothermic (h is positive), and entropy increases (s is positive) as the water molecules lose ordered arrangement. The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly freezes into slush.. Endothermic Reaction And Entropy.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction And Entropy Predict whether entropy change for a reaction is increasing or. When ice melts, the change is endothermic (h is positive), and entropy increases (s is positive) as the water molecules lose ordered arrangement. Some endothermic reactions are able to occur spontaneously at room temperature. Bond energies (the amount of energy that must be added in order to break a bond). Endothermic Reaction And Entropy.
From narodnatribuna.info
Endothermic Reactions Definition And Examples Endothermic Reaction And Entropy This shows how enthalpy is not the only driver of chemical. This condition describes an endothermic process that involves a decrease in system entropy. The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly freezes into slush. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as. Endothermic Reaction And Entropy.
From www.chemistrystudent.com
Total Entropy (ALevel) ChemistryStudent Endothermic Reaction And Entropy Some endothermic reactions are able to occur spontaneously at room temperature. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. Since entropy increases when temperature increases, the entropy of surroundings increases during an exothermic. This shows how enthalpy is not the only driver of chemical. In this case, δ g will be. Endothermic Reaction And Entropy.
From eduinput.com
Difference Between Endothermic And Exothermic Reactions Endothermic Reaction And Entropy The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly freezes into slush. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. Some endothermic reactions are able to occur spontaneously at room temperature. This shows how enthalpy is not the only. Endothermic Reaction And Entropy.
From mungfali.com
Endothermic Diagram With Labels Endothermic Reaction And Entropy This shows how enthalpy is not the only driver of chemical. Bond energies (the amount of energy that must be added in order to break a bond) are an. The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly freezes into slush. In this case, δ g will be. Endothermic Reaction And Entropy.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction And Entropy Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. This shows how enthalpy is not the only driver of chemical. Bond energies (the amount of energy that must be added in order to break a bond) are an. In this case, δ g will be positive regardless of the temperature. The reaction. Endothermic Reaction And Entropy.
From www.expii.com
Spontaneous and Nonspontaneous Reactions — Overview Expii Endothermic Reaction And Entropy Recall the meaning of exothermic and endothermic. When ice melts, the change is endothermic (h is positive), and entropy increases (s is positive) as the water molecules lose ordered arrangement. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. Since entropy increases when temperature increases, the entropy of surroundings increases during an. Endothermic Reaction And Entropy.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction And Entropy Some endothermic reactions are able to occur spontaneously at room temperature. Recall the meaning of exothermic and endothermic. Predict whether entropy change for a reaction is increasing or. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. Since entropy increases when temperature increases, the entropy of surroundings increases during an exothermic. When. Endothermic Reaction And Entropy.
From avopix.com
Endothermic and exothermic reaction diagram Royalty Free Stock Vector Endothermic Reaction And Entropy This condition describes an endothermic process that involves a decrease in system entropy. Since entropy increases when temperature increases, the entropy of surroundings increases during an exothermic. In this case, δ g will be positive regardless of the temperature. The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly. Endothermic Reaction And Entropy.
From socratic.org
How do I relate equilibrium constants to temperature change to find the Endothermic Reaction And Entropy Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. When ice melts, the change is endothermic (h is positive), and entropy increases (s is positive) as the water molecules lose ordered arrangement. Since entropy increases when temperature increases, the entropy of surroundings increases during an exothermic. Recall the meaning of exothermic and. Endothermic Reaction And Entropy.
From www.chegg.com
Endothermic reaction; decrease in entropy Calculate Endothermic Reaction And Entropy This condition describes an endothermic process that involves a decrease in system entropy. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. Recall the meaning of exothermic and endothermic. The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly freezes into. Endothermic Reaction And Entropy.
From stock.adobe.com
Enthalpy diagram for endothermic reactions Stock Vector Adobe Stock Endothermic Reaction And Entropy This shows how enthalpy is not the only driver of chemical. When ice melts, the change is endothermic (h is positive), and entropy increases (s is positive) as the water molecules lose ordered arrangement. Predict whether entropy change for a reaction is increasing or. In this case, δ g will be positive regardless of the temperature. Most endothermic reactions are. Endothermic Reaction And Entropy.
From khatiar1955a.altervista.org
The Difference Between Endothermic and Exothermic Reactions Endothermic Reaction And Entropy Since entropy increases when temperature increases, the entropy of surroundings increases during an exothermic. In this case, δ g will be positive regardless of the temperature. This shows how enthalpy is not the only driver of chemical. The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly freezes into. Endothermic Reaction And Entropy.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction And Entropy This condition describes an endothermic process that involves a decrease in system entropy. Bond energies (the amount of energy that must be added in order to break a bond) are an. Since entropy increases when temperature increases, the entropy of surroundings increases during an exothermic. When ice melts, the change is endothermic (h is positive), and entropy increases (s is. Endothermic Reaction And Entropy.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction And Entropy This condition describes an endothermic process that involves a decrease in system entropy. The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly freezes into slush. Since entropy increases when temperature increases, the entropy of surroundings increases during an exothermic. Some endothermic reactions are able to occur spontaneously at. Endothermic Reaction And Entropy.
From signalticket9.pythonanywhere.com
Simple Endothermic Reactions With Equations Reaction Balanced Equation Endothermic Reaction And Entropy Some endothermic reactions are able to occur spontaneously at room temperature. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. This shows how enthalpy is not the only driver of chemical. The reaction of barium hydroxide with ammonium thiocyanate is spontaneous but highly endothermic, so water, one product of the reaction, quickly. Endothermic Reaction And Entropy.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Endothermic Reaction And Entropy This shows how enthalpy is not the only driver of chemical. Recall the meaning of exothermic and endothermic. Some endothermic reactions are able to occur spontaneously at room temperature. Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. Bond energies (the amount of energy that must be added in order to break. Endothermic Reaction And Entropy.