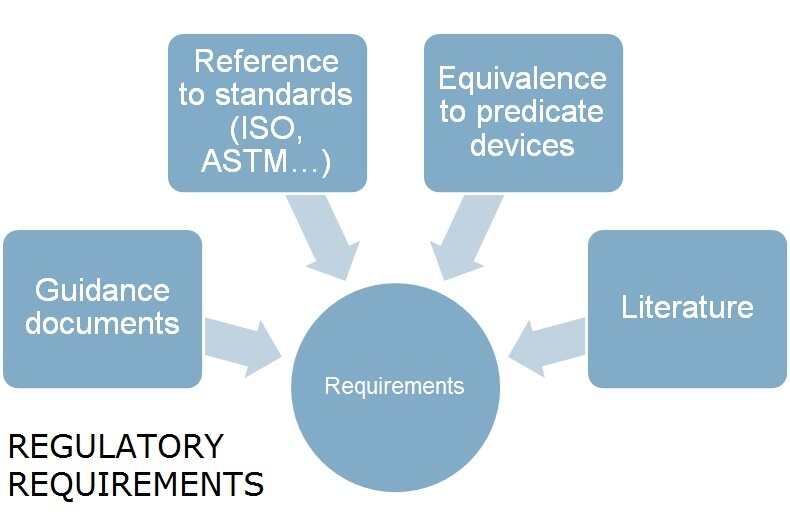
Lab Regulatory Requirements . It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. As a result, its requirements apply directly to laboratories that offer tests they developed themselves. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). 1.4 the laboratory management should ensure that all personnel. The fda is a us federal government regulatory agency. Regulatory and technological requirements and acceptability criteria. They regulate medical devices, including in vitro. There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain to the laboratory activities and.
from medinstitute.com
The fda is a us federal government regulatory agency. They regulate medical devices, including in vitro. As a result, its requirements apply directly to laboratories that offer tests they developed themselves. There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain to the laboratory activities and. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). Regulatory and technological requirements and acceptability criteria. 1.4 the laboratory management should ensure that all personnel. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories.
What do you do when regulatory requirements exceed reasonable
Lab Regulatory Requirements The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). As a result, its requirements apply directly to laboratories that offer tests they developed themselves. 1.4 the laboratory management should ensure that all personnel. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. Regulatory and technological requirements and acceptability criteria. They regulate medical devices, including in vitro. There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain to the laboratory activities and. The fda is a us federal government regulatory agency.
From www.lighthouselabservices.com
What Is a CLIA Lab Director and What Are Their Requirements? Lab Regulatory Requirements 1.4 the laboratory management should ensure that all personnel. The fda is a us federal government regulatory agency. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices. Lab Regulatory Requirements.
From www.slideserve.com
PPT Quality Assurance and Regulatory Compliance for Pharmaceutical Lab Regulatory Requirements The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain to the laboratory activities and. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies. Lab Regulatory Requirements.
From www.complianceonline.com
cGMP and GLP Regulations for Quality Control Labs An overview Lab Regulatory Requirements 1.4 the laboratory management should ensure that all personnel. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. The fda is a us federal government regulatory agency. As a result, its requirements apply directly to laboratories that offer tests they developed themselves. Regulatory and technological requirements and acceptability criteria.. Lab Regulatory Requirements.
From labtesting.wuxiapptec.com
Regulatory Pathways IND Requirement Variations Between U.S. FDA, OECD Lab Regulatory Requirements Regulatory and technological requirements and acceptability criteria. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. 1.4 the laboratory management should ensure that all personnel. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). There. Lab Regulatory Requirements.
From www.slideserve.com
PPT Regulatory Requirements for BE PowerPoint Presentation, free Lab Regulatory Requirements 1.4 the laboratory management should ensure that all personnel. They regulate medical devices, including in vitro. As a result, its requirements apply directly to laboratories that offer tests they developed themselves. There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain to the laboratory activities and. It defines the regulatory requirements for registration, licensing,. Lab Regulatory Requirements.
From blog.sierralabs.com
6 Regulatory Pathways to Bring Your Medical Device to Market Lab Regulatory Requirements There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain to the laboratory activities and. As a result, its requirements apply directly to laboratories that offer tests they developed themselves. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. They regulate medical devices,. Lab Regulatory Requirements.
From www.slideserve.com
PPT Regulatory Requirements for Bioanalytical Services PowerPoint Lab Regulatory Requirements 1.4 the laboratory management should ensure that all personnel. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). The fda is a us federal government regulatory agency. As a result, its requirements apply directly to laboratories that offer tests they developed themselves. Regulatory and technological requirements. Lab Regulatory Requirements.
From blog.sierralabs.com
ISO 13485 Regulatory Requirements on Medical Devices Lab Regulatory Requirements They regulate medical devices, including in vitro. The fda is a us federal government regulatory agency. As a result, its requirements apply directly to laboratories that offer tests they developed themselves. 1.4 the laboratory management should ensure that all personnel. Regulatory and technological requirements and acceptability criteria. The eu legislator is regulating laboratory developed tests for the first time with. Lab Regulatory Requirements.
From www.youtube.com
Regulatory requirement for approval of api, biologics and novel drugs Lab Regulatory Requirements It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. As a result, its requirements apply directly to laboratories that offer tests they developed themselves. 1.4 the laboratory management should ensure that all personnel. Regulatory and technological requirements and acceptability criteria. They regulate medical devices, including in vitro. The fda. Lab Regulatory Requirements.
From www.slideserve.com
PPT Regulatory Requirements for BE PowerPoint Presentation, free Lab Regulatory Requirements Regulatory and technological requirements and acceptability criteria. The fda is a us federal government regulatory agency. As a result, its requirements apply directly to laboratories that offer tests they developed themselves. They regulate medical devices, including in vitro. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. The eu. Lab Regulatory Requirements.
From dokumen.tips
(PDF) REGULATORY REQUIREMENTS FOR SOUTH AMERICA … requirements Lab Regulatory Requirements As a result, its requirements apply directly to laboratories that offer tests they developed themselves. Regulatory and technological requirements and acceptability criteria. 1.4 the laboratory management should ensure that all personnel. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. The fda is a us federal government regulatory agency.. Lab Regulatory Requirements.
From www.g2intelligence.com
Regulatory Roundup New laws, regulations, cases, and enforcement Lab Regulatory Requirements There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain to the laboratory activities and. Regulatory and technological requirements and acceptability criteria. 1.4 the laboratory management should ensure that all personnel. The fda is a us federal government regulatory agency. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories,. Lab Regulatory Requirements.
From blog.sierralabs.com
8 Regulatory Strategy Guidelines for Your Medical Device Lab Regulatory Requirements The fda is a us federal government regulatory agency. Regulatory and technological requirements and acceptability criteria. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. 1.4 the laboratory management should ensure that all personnel. The eu legislator is regulating laboratory developed tests for the first time with eu regulation. Lab Regulatory Requirements.
From training.nrhp.org
20230119 Regulatory Requirements Training and Education for Lab Regulatory Requirements They regulate medical devices, including in vitro. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. 1.4 the laboratory management should ensure that all personnel. As a result, its requirements apply directly to laboratories that offer tests they developed themselves. Regulatory and technological requirements and acceptability criteria. The eu. Lab Regulatory Requirements.
From www.researchgate.net
Comparative chart showing the regulatory requirements in USA, EU and Lab Regulatory Requirements As a result, its requirements apply directly to laboratories that offer tests they developed themselves. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. 1.4 the laboratory management should ensure that all personnel. There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain. Lab Regulatory Requirements.
From medinstitute.com
What do you do when regulatory requirements exceed reasonable Lab Regulatory Requirements As a result, its requirements apply directly to laboratories that offer tests they developed themselves. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). 1.4 the laboratory management should ensure that all personnel. There are a number of federal, state, and local laws, regulations, ordinances, and. Lab Regulatory Requirements.
From blog.sierralabs.com
ISO 13485 Regulatory Requirements on Medical Devices Lab Regulatory Requirements The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). 1.4 the laboratory management should ensure that all personnel. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. As a result, its requirements apply directly to. Lab Regulatory Requirements.
From nimonik.com
How to identify your regulatory requirements or variability in Lab Regulatory Requirements There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain to the laboratory activities and. Regulatory and technological requirements and acceptability criteria. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. 1.4 the laboratory management should ensure that all personnel. As a result,. Lab Regulatory Requirements.
From www.mlo-online.com
CLIA and regulatory readiness How can your lab always be ready? Lab Regulatory Requirements The fda is a us federal government regulatory agency. There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain to the laboratory activities and. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). It defines the regulatory requirements for registration,. Lab Regulatory Requirements.
From www.pharmabiotic.org
Regulatory Science & Tools PRI Lab Regulatory Requirements As a result, its requirements apply directly to laboratories that offer tests they developed themselves. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. 1.4 the laboratory management should ensure that all personnel. There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain. Lab Regulatory Requirements.
From www.researchgate.net
Comparison of regulatory requirements between generics and biosimilars Lab Regulatory Requirements The fda is a us federal government regulatory agency. Regulatory and technological requirements and acceptability criteria. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). As a result, its requirements apply directly to laboratories that offer tests they developed themselves. 1.4 the laboratory management should ensure. Lab Regulatory Requirements.
From www.apcerls.com
Safety & Regulatory requirements for Medical Devices APCER Life Sciences Lab Regulatory Requirements It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. They regulate medical devices, including in vitro. As a result, its requirements apply directly to laboratories that offer tests they developed themselves. There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain to the. Lab Regulatory Requirements.
From www.kewaunee.in
Designing a Laboratory Safety and Regulatory Requirements Kewaunee Lab Regulatory Requirements 1.4 the laboratory management should ensure that all personnel. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. There are a number of federal, state, and. Lab Regulatory Requirements.
From studylib.net
Regulatory Overview Table Lab Regulatory Requirements They regulate medical devices, including in vitro. As a result, its requirements apply directly to laboratories that offer tests they developed themselves. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in. Lab Regulatory Requirements.
From www.kewaunee.in
Laboratory Benches & Regulatory Standards Kewaunee Lab Regulatory Requirements The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). The fda is a us federal government regulatory agency. They regulate medical devices, including in vitro. There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain to the laboratory activities and.. Lab Regulatory Requirements.
From www.labcompare.com
MettlerToledo’s LabX software support for your lab’s regulatory Lab Regulatory Requirements Regulatory and technological requirements and acceptability criteria. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). They regulate medical devices, including in vitro. 1.4 the laboratory management should ensure that all personnel. There are a number of federal, state, and local laws, regulations, ordinances, and standards. Lab Regulatory Requirements.
From www.studocu.com
Regulatory requirements for drug approval Regulatory requirements for Lab Regulatory Requirements Regulatory and technological requirements and acceptability criteria. 1.4 the laboratory management should ensure that all personnel. The fda is a us federal government regulatory agency. They regulate medical devices, including in vitro. There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain to the laboratory activities and. As a result, its requirements apply directly. Lab Regulatory Requirements.
From blog.sierralabs.com
ISO 13485 Regulatory Requirements on Medical Devices Lab Regulatory Requirements It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). 1.4 the laboratory management should ensure that all personnel. There are a number of federal, state, and. Lab Regulatory Requirements.
From blog.medisolv.com
The Ultimate Regulatory Requirements Kit [DOWNLOAD] Lab Regulatory Requirements The fda is a us federal government regulatory agency. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. As a result, its requirements apply directly to laboratories that offer tests they developed themselves. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746. Lab Regulatory Requirements.
From www.researchgate.net
(PDF) An Update On Best Practices and Regulatory Requirements for the Lab Regulatory Requirements Regulatory and technological requirements and acceptability criteria. The fda is a us federal government regulatory agency. They regulate medical devices, including in vitro. There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain to the laboratory activities and. 1.4 the laboratory management should ensure that all personnel. It defines the regulatory requirements for registration,. Lab Regulatory Requirements.
From www.lansmont.com
Amgen meets regulatory requirement via FieldtoLab methodology Lansmont Lab Regulatory Requirements 1.4 the laboratory management should ensure that all personnel. They regulate medical devices, including in vitro. It defines the regulatory requirements for registration, licensing, certification and/or accreditation of health laboratories, and applies to all health laboratories. As a result, its requirements apply directly to laboratories that offer tests they developed themselves. Regulatory and technological requirements and acceptability criteria. The fda. Lab Regulatory Requirements.
From www.researchgate.net
Regulatory requirements for the Clinical Trial Imaging Management Lab Regulatory Requirements As a result, its requirements apply directly to laboratories that offer tests they developed themselves. They regulate medical devices, including in vitro. The fda is a us federal government regulatory agency. Regulatory and technological requirements and acceptability criteria. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices. Lab Regulatory Requirements.
From www.kewaunee.in
Designing BSL Labs Essential Requirements Kewaunee Lab Regulatory Requirements Regulatory and technological requirements and acceptability criteria. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). They regulate medical devices, including in vitro. There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain to the laboratory activities and. The fda. Lab Regulatory Requirements.
From sbnsoftware.com
Simple But Needed Lab Regulatory Requirements The fda is a us federal government regulatory agency. There are a number of federal, state, and local laws, regulations, ordinances, and standards that pertain to the laboratory activities and. Regulatory and technological requirements and acceptability criteria. As a result, its requirements apply directly to laboratories that offer tests they developed themselves. They regulate medical devices, including in vitro. 1.4. Lab Regulatory Requirements.
From www.slideshare.net
Regulatory requirement for drug approval Lab Regulatory Requirements 1.4 the laboratory management should ensure that all personnel. The eu legislator is regulating laboratory developed tests for the first time with eu regulation 2017/746 on in vitro diagnostic medical devices (ivdr). Regulatory and technological requirements and acceptability criteria. The fda is a us federal government regulatory agency. They regulate medical devices, including in vitro. It defines the regulatory requirements. Lab Regulatory Requirements.