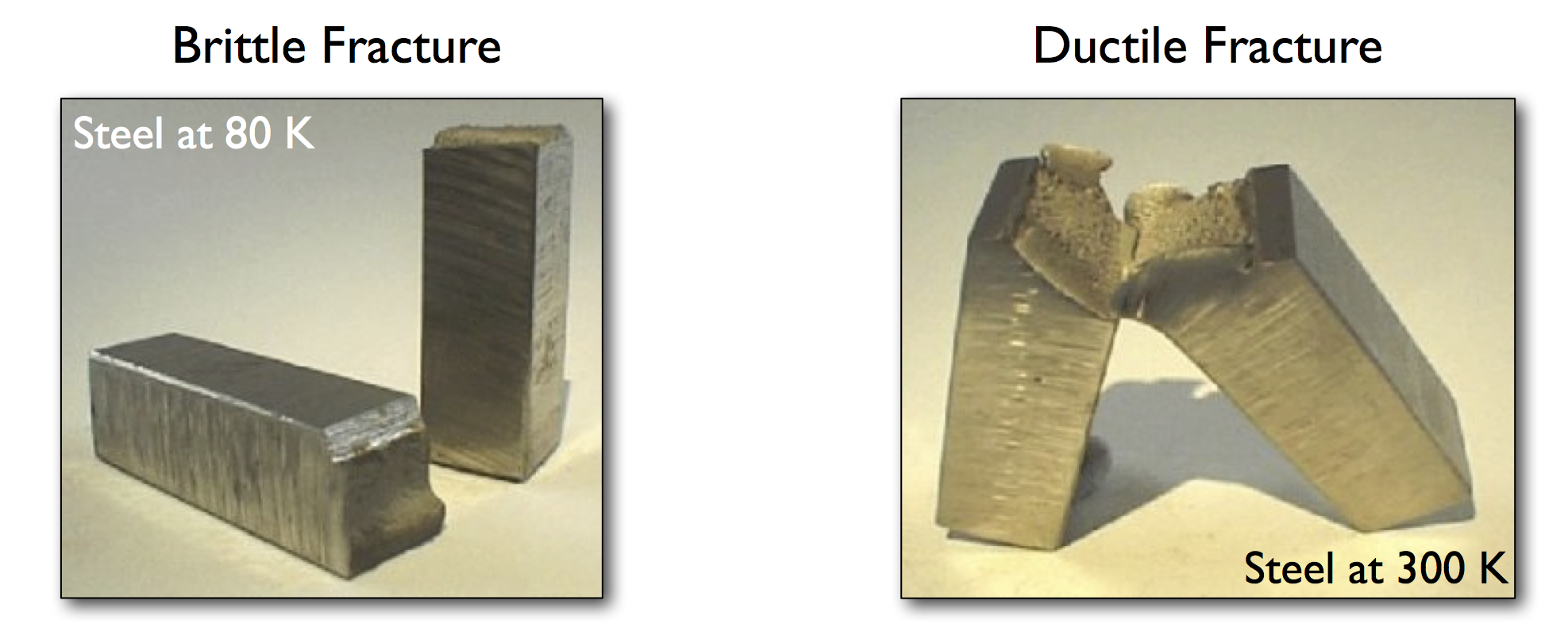
Crystalline And Brittle Example . for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. The properties of the different. Some substances, such as boron oxide (shown in figure 2),. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. examples of brittle materials. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. crystalline solids are the most common type of solids, whose structure consists of a regular, repeating pattern of.
from ceeqxbur.blob.core.windows.net
the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. crystalline solids are the most common type of solids, whose structure consists of a regular, repeating pattern of. for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. examples of brittle materials. Some substances, such as boron oxide (shown in figure 2),. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. The properties of the different. The strongest known substance in the universe, diamond is built of carbon atoms in such a close.
Brittle Substance Example at Terrilyn Radcliffe blog
Crystalline And Brittle Example crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. The properties of the different. crystalline solids are the most common type of solids, whose structure consists of a regular, repeating pattern of. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. examples of brittle materials. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. Some substances, such as boron oxide (shown in figure 2),.
From ceeqxbur.blob.core.windows.net
Brittle Substance Example at Terrilyn Radcliffe blog Crystalline And Brittle Example the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. The properties of the different. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a. Crystalline And Brittle Example.
From www.slideserve.com
PPT PROPERTIES OF MATTER PowerPoint Presentation, free download ID5969038 Crystalline And Brittle Example the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. Some substances, such as boron oxide (shown in figure 2),. examples of brittle materials. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. crystalline solids are the most common type of solids,. Crystalline And Brittle Example.
From www.researchgate.net
Brittle fracture in polycrystalline iridium (a) sample prepared from... Download Scientific Crystalline And Brittle Example examples of brittle materials. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. The properties of the different. the main types of crystalline solids are ionic solids, metallic solids, covalent. Crystalline And Brittle Example.
From www.slideserve.com
PPT Examples of Clastic Texture PowerPoint Presentation, free download ID2270968 Crystalline And Brittle Example crystalline solids are the most common type of solids, whose structure consists of a regular, repeating pattern of. examples of brittle materials. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. crystalline substances can be described by the types of particles in them and the types of chemical bonding. Crystalline And Brittle Example.
From exolxueyq.blob.core.windows.net
Brittleness Definition Explain at Mary Curtis blog Crystalline And Brittle Example The strongest known substance in the universe, diamond is built of carbon atoms in such a close. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. The properties of the different. crystalline substances can be described by the types of particles in them and the types of chemical bonding that. Crystalline And Brittle Example.
From insidescience.org
BRIEF A Brittle Crystal Flexible in the Dark Inside Science Crystalline And Brittle Example The properties of the different. crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. The strongest known. Crystalline And Brittle Example.
From www.slideserve.com
PPT CHAPTER 7 PowerPoint Presentation, free download ID4358912 Crystalline And Brittle Example The properties of the different. Some substances, such as boron oxide (shown in figure 2),. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. The strongest known substance in the universe,. Crystalline And Brittle Example.
From www.slideserve.com
PPT Crystalline Solids PowerPoint Presentation, free download ID2970209 Crystalline And Brittle Example the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. The properties of the different. additionally, these types of crystalline solids. Crystalline And Brittle Example.
From ibstudy.net
4.1 Ionic bonding and structure Wang's website Crystalline And Brittle Example for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. examples of brittle materials. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. Some substances, such as boron. Crystalline And Brittle Example.
From slideplayer.com
Ionic and Metallic Bonds ppt download Crystalline And Brittle Example additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. examples of brittle materials. crystalline solids are the most common type of solids, whose structure consists of a regular, repeating pattern. Crystalline And Brittle Example.
From chem.libretexts.org
12.1 Crystalline and Amorphous Solids Chemistry LibreTexts Crystalline And Brittle Example The strongest known substance in the universe, diamond is built of carbon atoms in such a close. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. crystalline substances can be. Crystalline And Brittle Example.
From blog.thepipingmart.com
Explain In Detail The Concept Of Brittleness ThePipingMart Blog Crystalline And Brittle Example The strongest known substance in the universe, diamond is built of carbon atoms in such a close. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. Some substances, such as boron oxide (shown in figure 2),. The properties of the different. for examples, candle waxes are amorphous solids composed of. Crystalline And Brittle Example.
From slideplayer.com
Chapter 12 Intermolecular Forces Liquids, Solids, and Phase Changes. ppt download Crystalline And Brittle Example crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. Some substances, such as boron oxide (shown in figure 2),. examples of brittle materials. The properties of the different. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. The. Crystalline And Brittle Example.
From slideplayer.com
Characteristics and Properties ppt download Crystalline And Brittle Example The properties of the different. crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. examples of brittle materials. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. The strongest known substance in the universe, diamond is built of. Crystalline And Brittle Example.
From dxofcmjzd.blob.core.windows.net
Crystalline Definition Geology at Marion Lappin blog Crystalline And Brittle Example crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. examples of brittle. Crystalline And Brittle Example.
From www.slideserve.com
PPT Giant Ionic Structures PowerPoint Presentation, free download ID663487 Crystalline And Brittle Example The properties of the different. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. Some substances, such as boron oxide (shown in figure 2),. examples of brittle materials. additionally, these types of. Crystalline And Brittle Example.
From chem.libretexts.org
12.4 The Fundamental Types of Crystalline Solids Chemistry LibreTexts Crystalline And Brittle Example crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. crystalline solids are the most common type of solids, whose structure consists of a regular, repeating pattern of. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point.. Crystalline And Brittle Example.
From slideplayer.com
Ch. 7 LT1 Ionic Bonding and Metals ppt download Crystalline And Brittle Example The properties of the different. crystalline solids are the most common type of solids, whose structure consists of a regular, repeating pattern of. examples of brittle materials. for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. Some. Crystalline And Brittle Example.
From howforkids.com
Examples of Brittle Materials HowForKids Crystalline And Brittle Example crystalline solids are the most common type of solids, whose structure consists of a regular, repeating pattern of. crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point.. Crystalline And Brittle Example.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation, free download ID1130121 Crystalline And Brittle Example examples of brittle materials. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. the main types of. Crystalline And Brittle Example.
From www.askiitians.com
Classification of Crystalline Solids Study Material for IIT JEE askIITians Crystalline And Brittle Example crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. crystalline solids are the most common type of solids, whose structure consists of a regular, repeating pattern of.. Crystalline And Brittle Example.
From www.slideserve.com
PPT Fracture Behavior of Bulk Crystalline Materials PowerPoint Presentation ID3032093 Crystalline And Brittle Example examples of brittle materials. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. The strongest known substance in the universe, diamond is built of carbon atoms in such a close.. Crystalline And Brittle Example.
From howforkids.com
15 Examples of Brittle Materials HowForKids Crystalline And Brittle Example examples of brittle materials. for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. crystalline solids are the most common. Crystalline And Brittle Example.
From www.britannica.com
Bodycentred cubic structure crystalline form Britannica Crystalline And Brittle Example for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. Some substances, such as boron oxide (shown in figure 2),. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. examples of brittle materials. additionally, these types of crystalline solids are generally hard, brittle, and tend to. Crystalline And Brittle Example.
From www.youtube.com
Metallic, Ionic, and Molecular Solids Explained with Examples! YouTube Crystalline And Brittle Example Some substances, such as boron oxide (shown in figure 2),. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. crystalline solids are the most common type of solids, whose structure consists of a regular, repeating pattern of. additionally, these types of crystalline solids are generally hard, brittle, and tend to. Crystalline And Brittle Example.
From ceeqxbur.blob.core.windows.net
Brittle Substance Example at Terrilyn Radcliffe blog Crystalline And Brittle Example crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. examples of brittle materials. The strongest known substance in the universe, diamond is built of carbon atoms in. Crystalline And Brittle Example.
From www.slideserve.com
PPT Crystallinity in Polymers PowerPoint Presentation, free download ID3117708 Crystalline And Brittle Example The properties of the different. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. crystalline substances can be described by the types of particles in them and the types of chemical bonding that. Crystalline And Brittle Example.
From eduinput.com
Crystalline solids Properties, types, examples use Crystalline And Brittle Example The properties of the different. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. The strongest known substance in the universe, diamond is built of carbon atoms in such a close.. Crystalline And Brittle Example.
From ceizlash.blob.core.windows.net
Brittle Definition And Example at Shirley Love blog Crystalline And Brittle Example the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. Some substances, such as boron oxide (shown in figure 2),. for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. crystalline substances can be described by the types of particles in them and the types of chemical bonding. Crystalline And Brittle Example.
From firstyearengineer.com
Crystal Structure the first year engineer Crystalline And Brittle Example The properties of the different. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. Some substances, such as boron oxide (shown in figure 2),. examples of brittle materials. for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. the main types of crystalline solids. Crystalline And Brittle Example.
From ceizlash.blob.core.windows.net
Brittle Definition And Example at Shirley Love blog Crystalline And Brittle Example The properties of the different. crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. crystalline solids are the most common type of solids, whose structure consists of a regular, repeating pattern of. The strongest. Crystalline And Brittle Example.
From chem.libretexts.org
Crystalline Solid Structures Chemistry LibreTexts Crystalline And Brittle Example crystalline solids are the most common type of solids, whose structure consists of a regular, repeating pattern of. examples of brittle materials. Some substances, such as boron oxide (shown in figure 2),. crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. for examples, candle waxes. Crystalline And Brittle Example.
From slideplayer.com
Materials and Manufacturing processes ppt download Crystalline And Brittle Example additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. The properties of the different. The strongest known substance in the universe, diamond is built of carbon atoms in such a close. examples of brittle materials. for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules.. Crystalline And Brittle Example.
From www.chegg.com
Solved Properties Example 2. Crystalline Solids come in 4 Crystalline And Brittle Example crystalline substances can be described by the types of particles in them and the types of chemical bonding that take. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. Some substances, such as boron oxide (shown in. Crystalline And Brittle Example.
From learn.careers360.com
Show the structure of crystalline, polycrystalline and amorphous Crystalline And Brittle Example The properties of the different. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. additionally, these types of crystalline solids are generally hard, brittle, and tend to show a very high melting point. for examples, candle waxes are amorphous solids composed of large hydrocarbon molecules. Some substances, such as. Crystalline And Brittle Example.