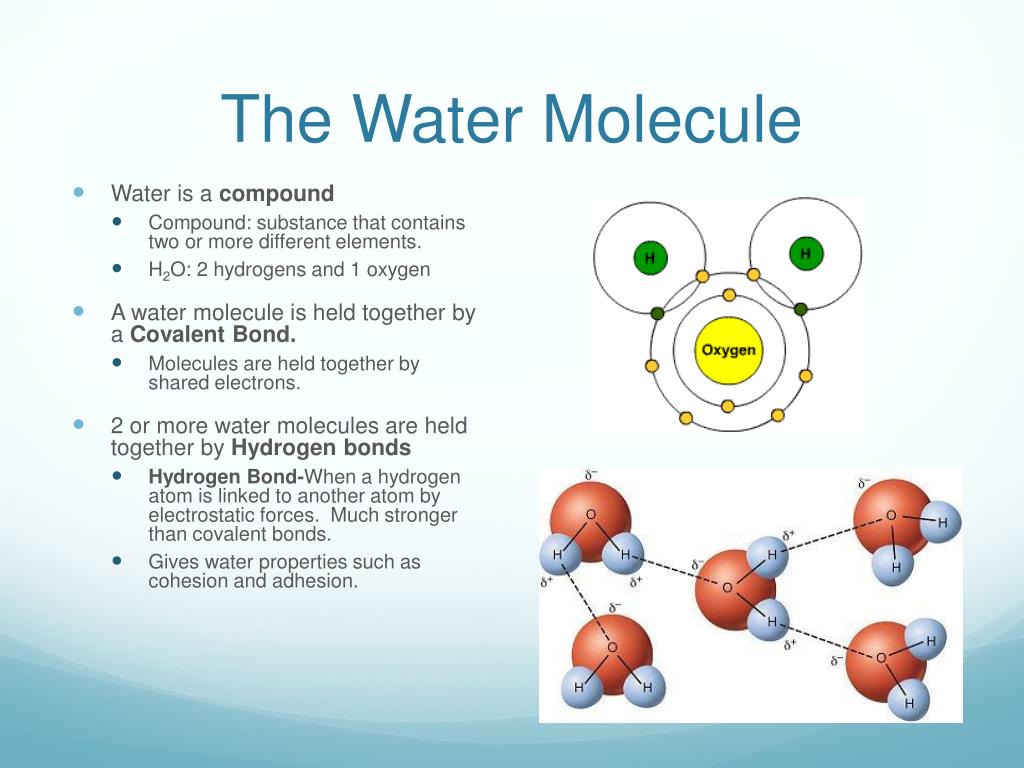
Water's Unique Density . Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation). water has many unique properties that allow it to sustain life. the polarity of water and its ability to hydrogen bond contributes to its unique properties. Normally, liquids become increasingly dense as they are cooled down, but water reaches a. what causes water's density to be unusual? Without it, life as we know it simply would not exist. When water freezes at 0°c (read. Ice is less dense than liquid water due to spaces in the intermolecular structure of ice not present in water. one of the weirdest aspects of water is its unusual density. The polarity of the water molecule and its resulting. Water's density is strange because it peaks at 4°c, unlike most liquids. the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense than liquid. the mw model is computationaly efficient and gives the energetics, structure, and density of liquid. This particular clip talks about how ice is less dense than liquid water.
from printableuklanjalqb.z22.web.core.windows.net
Water's density is strange because it peaks at 4°c, unlike most liquids. one of the weirdest aspects of water is its unusual density. the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense than liquid. The polarity of the water molecule and its resulting. Ice is less dense than liquid water due to spaces in the intermolecular structure of ice not present in water. the polarity of water and its ability to hydrogen bond contributes to its unique properties. This particular clip talks about how ice is less dense than liquid water. Without it, life as we know it simply would not exist. Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation). When water freezes at 0°c (read.
Properties Of Water Explained
Water's Unique Density Without it, life as we know it simply would not exist. Without it, life as we know it simply would not exist. what causes water's density to be unusual? one of the weirdest aspects of water is its unusual density. Ice is less dense than liquid water due to spaces in the intermolecular structure of ice not present in water. Normally, liquids become increasingly dense as they are cooled down, but water reaches a. the mw model is computationaly efficient and gives the energetics, structure, and density of liquid. When water freezes at 0°c (read. Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation). the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense than liquid. This particular clip talks about how ice is less dense than liquid water. The polarity of the water molecule and its resulting. Water's density is strange because it peaks at 4°c, unlike most liquids. water has many unique properties that allow it to sustain life. the polarity of water and its ability to hydrogen bond contributes to its unique properties.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID477327 Water's Unique Density what causes water's density to be unusual? This particular clip talks about how ice is less dense than liquid water. the mw model is computationaly efficient and gives the energetics, structure, and density of liquid. Ice is less dense than liquid water due to spaces in the intermolecular structure of ice not present in water. water has. Water's Unique Density.
From www.youtube.com
Unique Properties of Water YouTube Water's Unique Density Water's density is strange because it peaks at 4°c, unlike most liquids. water has many unique properties that allow it to sustain life. This particular clip talks about how ice is less dense than liquid water. The polarity of the water molecule and its resulting. one of the weirdest aspects of water is its unusual density. the. Water's Unique Density.
From www.wikihow.com
How to Find the Density of Water 10 Steps (with Pictures) Water's Unique Density Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation). the mw model is computationaly efficient and gives the energetics, structure, and density of liquid. the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense than liquid. Ice is less dense than liquid water due to spaces. Water's Unique Density.
From www.slideshare.net
Unique properties of water overview Water's Unique Density water has many unique properties that allow it to sustain life. what causes water's density to be unusual? Normally, liquids become increasingly dense as they are cooled down, but water reaches a. This particular clip talks about how ice is less dense than liquid water. Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation).. Water's Unique Density.
From www.nagwa.com
Lesson Video Properties of Water Nagwa Water's Unique Density Ice is less dense than liquid water due to spaces in the intermolecular structure of ice not present in water. the mw model is computationaly efficient and gives the energetics, structure, and density of liquid. what causes water's density to be unusual? When water freezes at 0°c (read. Ionic solute molecules are hydrated (surrounded by solvent molecules in. Water's Unique Density.
From slideplayer.com
The Unique Properties of Water ppt download Water's Unique Density When water freezes at 0°c (read. the mw model is computationaly efficient and gives the energetics, structure, and density of liquid. what causes water's density to be unusual? Water's density is strange because it peaks at 4°c, unlike most liquids. This particular clip talks about how ice is less dense than liquid water. one of the weirdest. Water's Unique Density.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID1616261 Water's Unique Density what causes water's density to be unusual? the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense than liquid. Without it, life as we know it simply would not exist. Ice is less dense than liquid water due to spaces in the intermolecular structure of ice not present in water.. Water's Unique Density.
From www.slideserve.com
PPT Water’s Unique Properties PowerPoint Presentation, free download ID2200855 Water's Unique Density The polarity of the water molecule and its resulting. water has many unique properties that allow it to sustain life. the mw model is computationaly efficient and gives the energetics, structure, and density of liquid. Without it, life as we know it simply would not exist. the polarity of water and its ability to hydrogen bond contributes. Water's Unique Density.
From www.sciencelearn.org.nz
Investigating water density — Science Learning Hub Water's Unique Density water has many unique properties that allow it to sustain life. one of the weirdest aspects of water is its unusual density. Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation). what causes water's density to be unusual? This particular clip talks about how ice is less dense than liquid water. Ice is. Water's Unique Density.
From printablepattiarajk.z22.web.core.windows.net
Properties Of Water Explained Water's Unique Density the polarity of water and its ability to hydrogen bond contributes to its unique properties. what causes water's density to be unusual? Normally, liquids become increasingly dense as they are cooled down, but water reaches a. When water freezes at 0°c (read. one of the weirdest aspects of water is its unusual density. water has many. Water's Unique Density.
From student-tutor.com
Understanding the Density of Water StudentTutor Education Blog Water's Unique Density one of the weirdest aspects of water is its unusual density. the polarity of water and its ability to hydrogen bond contributes to its unique properties. Normally, liquids become increasingly dense as they are cooled down, but water reaches a. Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation). When water freezes at 0°c. Water's Unique Density.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID524711 Water's Unique Density what causes water's density to be unusual? the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense than liquid. Without it, life as we know it simply would not exist. water has many unique properties that allow it to sustain life. Normally, liquids become increasingly dense as they are. Water's Unique Density.
From slideplayer.com
Title Water’s Unique Properties Allow Life to Exist on Earth. ppt download Water's Unique Density When water freezes at 0°c (read. Water's density is strange because it peaks at 4°c, unlike most liquids. the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense than liquid. This particular clip talks about how ice is less dense than liquid water. Normally, liquids become increasingly dense as they are. Water's Unique Density.
From www.slideserve.com
PPT Unique properties of Water PowerPoint Presentation, free download ID2883875 Water's Unique Density the mw model is computationaly efficient and gives the energetics, structure, and density of liquid. what causes water's density to be unusual? Normally, liquids become increasingly dense as they are cooled down, but water reaches a. the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense than liquid. Without. Water's Unique Density.
From printableuklanjalqb.z22.web.core.windows.net
Properties Of Water Explained Water's Unique Density what causes water's density to be unusual? Normally, liquids become increasingly dense as they are cooled down, but water reaches a. water has many unique properties that allow it to sustain life. When water freezes at 0°c (read. The polarity of the water molecule and its resulting. Water's density is strange because it peaks at 4°c, unlike most. Water's Unique Density.
From www.expii.com
Unique Densities (Water) — Properties & Examples Expii Water's Unique Density Normally, liquids become increasingly dense as they are cooled down, but water reaches a. the mw model is computationaly efficient and gives the energetics, structure, and density of liquid. Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation). water has many unique properties that allow it to sustain life. the open structure of. Water's Unique Density.
From slideplayer.com
The Unique Properties of Water ppt download Water's Unique Density the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense than liquid. When water freezes at 0°c (read. water has many unique properties that allow it to sustain life. Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation). what causes water's density to be unusual?. Water's Unique Density.
From www.slideserve.com
PPT Chemistry Of Life PowerPoint Presentation, free download ID6225405 Water's Unique Density the mw model is computationaly efficient and gives the energetics, structure, and density of liquid. Water's density is strange because it peaks at 4°c, unlike most liquids. Ice is less dense than liquid water due to spaces in the intermolecular structure of ice not present in water. This particular clip talks about how ice is less dense than liquid. Water's Unique Density.
From www.slideshare.net
Water properties Water's Unique Density Water's density is strange because it peaks at 4°c, unlike most liquids. the mw model is computationaly efficient and gives the energetics, structure, and density of liquid. water has many unique properties that allow it to sustain life. what causes water's density to be unusual? Without it, life as we know it simply would not exist. This. Water's Unique Density.
From www.slideserve.com
PPT Water’s Unique Properties PowerPoint Presentation, free download ID2200855 Water's Unique Density The polarity of the water molecule and its resulting. water has many unique properties that allow it to sustain life. When water freezes at 0°c (read. the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense than liquid. Without it, life as we know it simply would not exist. . Water's Unique Density.
From studylib.net
Lesson 1 Unique Properties of Water Water's Unique Density When water freezes at 0°c (read. what causes water's density to be unusual? The polarity of the water molecule and its resulting. Without it, life as we know it simply would not exist. the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense than liquid. the mw model is. Water's Unique Density.
From www.slideserve.com
PPT Water’s Unique Properties PowerPoint Presentation, free download ID2200855 Water's Unique Density When water freezes at 0°c (read. Normally, liquids become increasingly dense as they are cooled down, but water reaches a. This particular clip talks about how ice is less dense than liquid water. Without it, life as we know it simply would not exist. Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation). the polarity. Water's Unique Density.
From webapi.bu.edu
💣 3 properties of water. What are the 6 properties of water examples?. 20221019 Water's Unique Density one of the weirdest aspects of water is its unusual density. Without it, life as we know it simply would not exist. water has many unique properties that allow it to sustain life. Water's density is strange because it peaks at 4°c, unlike most liquids. the mw model is computationaly efficient and gives the energetics, structure, and. Water's Unique Density.
From www.slideshare.net
02 Properties of Water Water's Unique Density water has many unique properties that allow it to sustain life. Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation). Without it, life as we know it simply would not exist. one of the weirdest aspects of water is its unusual density. what causes water's density to be unusual? Ice is less dense. Water's Unique Density.
From owlcation.com
5 Properties of Water Owlcation Water's Unique Density Normally, liquids become increasingly dense as they are cooled down, but water reaches a. The polarity of the water molecule and its resulting. water has many unique properties that allow it to sustain life. the polarity of water and its ability to hydrogen bond contributes to its unique properties. the mw model is computationaly efficient and gives. Water's Unique Density.
From www.slideshare.net
Unique Properties Of Water Water's Unique Density Without it, life as we know it simply would not exist. the mw model is computationaly efficient and gives the energetics, structure, and density of liquid. water has many unique properties that allow it to sustain life. the polarity of water and its ability to hydrogen bond contributes to its unique properties. one of the weirdest. Water's Unique Density.
From www.slideshare.net
Unique properties of water overview Water's Unique Density Water's density is strange because it peaks at 4°c, unlike most liquids. water has many unique properties that allow it to sustain life. When water freezes at 0°c (read. This particular clip talks about how ice is less dense than liquid water. Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation). Ice is less dense. Water's Unique Density.
From hydroservice.com
The Unique and Unusual Properties of Water Hydro Services Water's Unique Density the polarity of water and its ability to hydrogen bond contributes to its unique properties. what causes water's density to be unusual? Normally, liquids become increasingly dense as they are cooled down, but water reaches a. When water freezes at 0°c (read. water has many unique properties that allow it to sustain life. one of the. Water's Unique Density.
From studylib.net
Introduction to the Properties of Water What Makes Water So Special Water's Unique Density water has many unique properties that allow it to sustain life. Water's density is strange because it peaks at 4°c, unlike most liquids. the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense than liquid. This particular clip talks about how ice is less dense than liquid water. Ionic solute. Water's Unique Density.
From www.slideshare.net
Unique Properties Of Water Water's Unique Density the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense than liquid. Normally, liquids become increasingly dense as they are cooled down, but water reaches a. what causes water's density to be unusual? the mw model is computationaly efficient and gives the energetics, structure, and density of liquid. . Water's Unique Density.
From www.slideshare.net
Properties of water Water's Unique Density When water freezes at 0°c (read. This particular clip talks about how ice is less dense than liquid water. what causes water's density to be unusual? Water's density is strange because it peaks at 4°c, unlike most liquids. The polarity of the water molecule and its resulting. Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific. Water's Unique Density.
From www.slideserve.com
PPT Intro to Water PowerPoint Presentation, free download ID1756675 Water's Unique Density the polarity of water and its ability to hydrogen bond contributes to its unique properties. Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation). The polarity of the water molecule and its resulting. Without it, life as we know it simply would not exist. the open structure of ice that allows for maximum hydrogen. Water's Unique Density.
From animalia-life.club
Properties Of Water Polarity Water's Unique Density Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation). water has many unique properties that allow it to sustain life. Normally, liquids become increasingly dense as they are cooled down, but water reaches a. what causes water's density to be unusual? the polarity of water and its ability to hydrogen bond contributes to. Water's Unique Density.
From www.slideserve.com
PPT CEE 210 Environmental Biology for Engineers PowerPoint Presentation ID6806229 Water's Unique Density the mw model is computationaly efficient and gives the energetics, structure, and density of liquid. Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation). The polarity of the water molecule and its resulting. water has many unique properties that allow it to sustain life. Water's density is strange because it peaks at 4°c, unlike. Water's Unique Density.
From studylib.net
Water*s Unique Properties Water's Unique Density This particular clip talks about how ice is less dense than liquid water. The polarity of the water molecule and its resulting. water has many unique properties that allow it to sustain life. Ionic solute molecules are hydrated (surrounded by solvent molecules in a specific orientation). what causes water's density to be unusual? Water's density is strange because. Water's Unique Density.