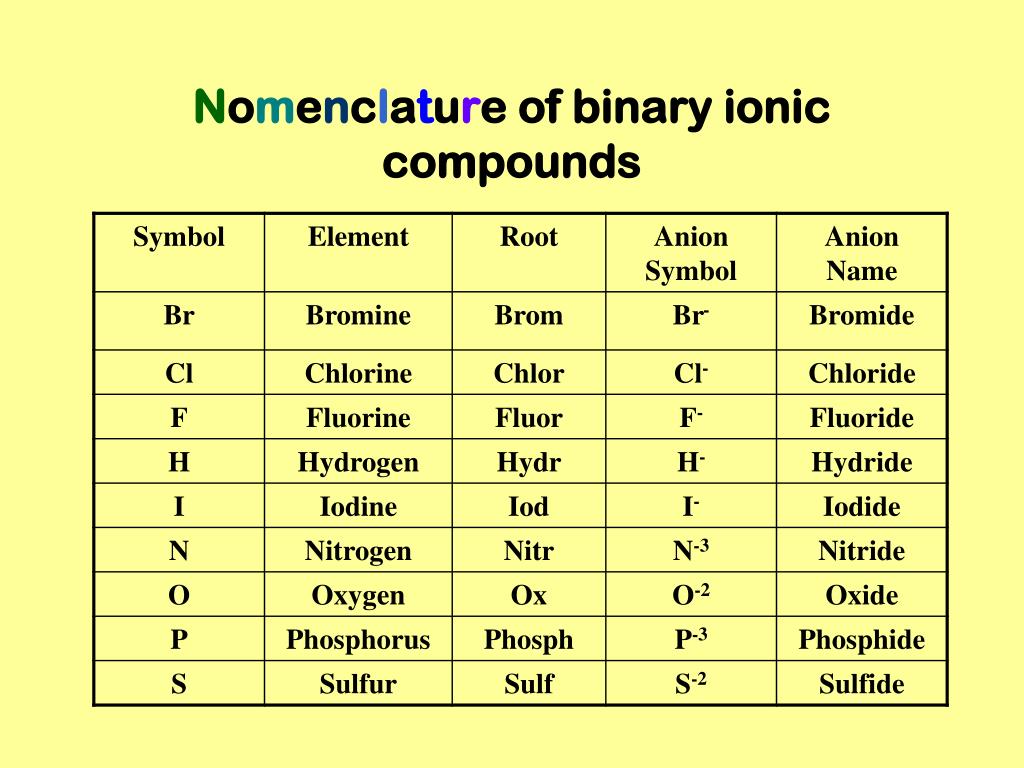
Why Is It Called A Binary Ionic Compound . Although a binary compound only contains two elements, it can contain. A metal (which forms the cations) and a nonmetal (which forms the anions). Ions of main group elements were discussed thoroughly in. The charge of the ions can be determined by their location within the periodic table. A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. The metal cation is named first, followed by the nonmetal anion. Binary ionic compounds are composed of just two elements: Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. Many compounds, particularly those that have been known for a relatively long time, have more than one name: It is necessary, however, for a group 16 or 17. In chemistry, a binary compound is a chemical compound consisting of exactly two different elements. For example, nacl is a binary ionic compound.
from www.slideserve.com
Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. Binary ionic compounds are composed of just two elements: It is necessary, however, for a group 16 or 17. Although a binary compound only contains two elements, it can contain. Many compounds, particularly those that have been known for a relatively long time, have more than one name: For example, nacl is a binary ionic compound. In chemistry, a binary compound is a chemical compound consisting of exactly two different elements. A metal (which forms the cations) and a nonmetal (which forms the anions). A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. The metal cation is named first, followed by the nonmetal anion.
PPT NAMING IONIC COMPOUNDS PowerPoint Presentation, free download
Why Is It Called A Binary Ionic Compound A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. The metal cation is named first, followed by the nonmetal anion. The charge of the ions can be determined by their location within the periodic table. A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. For example, nacl is a binary ionic compound. It is necessary, however, for a group 16 or 17. Binary ionic compounds are composed of just two elements: A metal (which forms the cations) and a nonmetal (which forms the anions). Although a binary compound only contains two elements, it can contain. Many compounds, particularly those that have been known for a relatively long time, have more than one name: Ions of main group elements were discussed thoroughly in. In chemistry, a binary compound is a chemical compound consisting of exactly two different elements.
From www.slideserve.com
PPT Ionic Compounds Formula to Name PowerPoint Presentation, free Why Is It Called A Binary Ionic Compound A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. The metal cation is named first, followed by the nonmetal anion. Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. In chemistry, a binary compound is a chemical compound consisting of exactly two different elements. Binary. Why Is It Called A Binary Ionic Compound.
From study.com
How to Name Binary Ionic Compounds Chemistry Why Is It Called A Binary Ionic Compound Binary ionic compounds are composed of just two elements: Many compounds, particularly those that have been known for a relatively long time, have more than one name: Although a binary compound only contains two elements, it can contain. The charge of the ions can be determined by their location within the periodic table. Therefore, presence of a particular nonmetal does. Why Is It Called A Binary Ionic Compound.
From genderi.org
The Nomenclature of Binary Compounds Formula of Binary Compounds Why Is It Called A Binary Ionic Compound A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. The charge of the ions can be determined by their location within the periodic table. Although a binary compound only contains two elements, it can contain. For example, nacl is a binary ionic compound. The metal cation is named first, followed by. Why Is It Called A Binary Ionic Compound.
From www.slideserve.com
PPT IONIC COMPOUNDS Names and Formulas PowerPoint Presentation, free Why Is It Called A Binary Ionic Compound Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. It is necessary, however, for a group 16 or 17. In chemistry, a binary compound is a chemical compound consisting of exactly two different elements. Many compounds,. Why Is It Called A Binary Ionic Compound.
From www.slideserve.com
PPT Naming Chemical Compounds PowerPoint Presentation, free download Why Is It Called A Binary Ionic Compound Binary ionic compounds are composed of just two elements: The metal cation is named first, followed by the nonmetal anion. Although a binary compound only contains two elements, it can contain. Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. Ions of main group elements were discussed thoroughly in. A metal (which forms the. Why Is It Called A Binary Ionic Compound.
From www.slideserve.com
PPT Naming Chemical Compounds PowerPoint Presentation, free download Why Is It Called A Binary Ionic Compound Many compounds, particularly those that have been known for a relatively long time, have more than one name: A metal (which forms the cations) and a nonmetal (which forms the anions). It is necessary, however, for a group 16 or 17. In chemistry, a binary compound is a chemical compound consisting of exactly two different elements. A binary ionic compound. Why Is It Called A Binary Ionic Compound.
From slideplayer.com
Ionic Compounds Naming ppt download Why Is It Called A Binary Ionic Compound It is necessary, however, for a group 16 or 17. The charge of the ions can be determined by their location within the periodic table. Although a binary compound only contains two elements, it can contain. A metal (which forms the cations) and a nonmetal (which forms the anions). In chemistry, a binary compound is a chemical compound consisting of. Why Is It Called A Binary Ionic Compound.
From www.slideserve.com
PPT Binary Ionic Compounds (Type I) PowerPoint Presentation, free Why Is It Called A Binary Ionic Compound Ions of main group elements were discussed thoroughly in. Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. The metal cation is named first, followed by the nonmetal anion. The charge of the ions can be. Why Is It Called A Binary Ionic Compound.
From www.slideserve.com
PPT Writing Formulas for Binary Ionic Compounds PowerPoint Why Is It Called A Binary Ionic Compound A metal (which forms the cations) and a nonmetal (which forms the anions). For example, nacl is a binary ionic compound. Many compounds, particularly those that have been known for a relatively long time, have more than one name: It is necessary, however, for a group 16 or 17. The metal cation is named first, followed by the nonmetal anion.. Why Is It Called A Binary Ionic Compound.
From slideplayer.com
Ionic Compounds Naming ppt download Why Is It Called A Binary Ionic Compound For example, nacl is a binary ionic compound. A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. Although a binary compound only contains two elements, it can contain. Binary ionic compounds are composed of just two elements: The metal cation is named first, followed by the nonmetal anion. Therefore, presence of. Why Is It Called A Binary Ionic Compound.
From www.youtube.com
What are Binary Ionic Compounds Formation and Naming YouTube Why Is It Called A Binary Ionic Compound Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. Binary ionic compounds are composed of just two elements: The charge of the ions can be determined by their location within the periodic table. For example, nacl is a binary ionic compound. The metal cation is named first, followed by the nonmetal anion. A metal. Why Is It Called A Binary Ionic Compound.
From www.slideserve.com
PPT Writing Formulas for Binary Ionic Compounds PowerPoint Why Is It Called A Binary Ionic Compound A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. It is necessary, however, for a group 16 or 17. In chemistry, a binary compound is a chemical compound consisting of exactly two different elements. Ions of main group elements were discussed thoroughly in. A metal (which forms the cations) and a. Why Is It Called A Binary Ionic Compound.
From www.slideserve.com
PPT The Nomenclature of Binary Compounds PowerPoint Presentation ID Why Is It Called A Binary Ionic Compound It is necessary, however, for a group 16 or 17. In chemistry, a binary compound is a chemical compound consisting of exactly two different elements. The charge of the ions can be determined by their location within the periodic table. Although a binary compound only contains two elements, it can contain. The metal cation is named first, followed by the. Why Is It Called A Binary Ionic Compound.
From sciencenotes.org
What Is a Binary Compound? Definition and Examples Why Is It Called A Binary Ionic Compound The charge of the ions can be determined by their location within the periodic table. For example, nacl is a binary ionic compound. Many compounds, particularly those that have been known for a relatively long time, have more than one name: Binary ionic compounds are composed of just two elements: A binary ionic compound is a compound composed of a. Why Is It Called A Binary Ionic Compound.
From www.slideserve.com
PPT Naming Binary Ionic Compounds PowerPoint Presentation, free Why Is It Called A Binary Ionic Compound Ions of main group elements were discussed thoroughly in. It is necessary, however, for a group 16 or 17. Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. The charge of the ions can be determined by their location within the periodic table. Binary ionic compounds are composed of just two elements: A metal. Why Is It Called A Binary Ionic Compound.
From slideplayer.com
Ionic Compounds. ppt download Why Is It Called A Binary Ionic Compound For example, nacl is a binary ionic compound. The charge of the ions can be determined by their location within the periodic table. Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. In chemistry, a binary compound is a chemical compound consisting of exactly two different elements. It is necessary, however, for a group. Why Is It Called A Binary Ionic Compound.
From slideplayer.com
Table of Contents Chemical Names and Formulas of ionic compounds ppt Why Is It Called A Binary Ionic Compound Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. The metal cation is named first, followed by the nonmetal anion. Ions of main group elements were discussed thoroughly in. Although a binary compound only contains two elements, it can contain. Many compounds, particularly those that have been known for a relatively long time, have. Why Is It Called A Binary Ionic Compound.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation, free download ID Why Is It Called A Binary Ionic Compound Many compounds, particularly those that have been known for a relatively long time, have more than one name: In chemistry, a binary compound is a chemical compound consisting of exactly two different elements. It is necessary, however, for a group 16 or 17. Ions of main group elements were discussed thoroughly in. A binary ionic compound is a compound composed. Why Is It Called A Binary Ionic Compound.
From www.slideserve.com
PPT Aim How do you name ionic compounds? PowerPoint Presentation Why Is It Called A Binary Ionic Compound Binary ionic compounds are composed of just two elements: For example, nacl is a binary ionic compound. Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. Ions of main group elements were discussed thoroughly in. The metal cation is named first, followed by the nonmetal anion. Although a binary compound only contains two elements,. Why Is It Called A Binary Ionic Compound.
From slideplayer.com
LECTURE 5 IONS & IONIC COMPOUNDS. ppt download Why Is It Called A Binary Ionic Compound Although a binary compound only contains two elements, it can contain. A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. A metal (which forms the cations) and a nonmetal (which forms the anions). Ions of main group elements were discussed thoroughly in. Therefore, presence of a particular nonmetal does not guarantee. Why Is It Called A Binary Ionic Compound.
From www.slideserve.com
PPT Writing Formulas for Binary Ionic Compounds PowerPoint Why Is It Called A Binary Ionic Compound For example, nacl is a binary ionic compound. Ions of main group elements were discussed thoroughly in. Although a binary compound only contains two elements, it can contain. The metal cation is named first, followed by the nonmetal anion. It is necessary, however, for a group 16 or 17. In chemistry, a binary compound is a chemical compound consisting of. Why Is It Called A Binary Ionic Compound.
From www.youtube.com
Naming and Formulas for Binary Ionic Compounds YouTube Why Is It Called A Binary Ionic Compound The metal cation is named first, followed by the nonmetal anion. In chemistry, a binary compound is a chemical compound consisting of exactly two different elements. It is necessary, however, for a group 16 or 17. Many compounds, particularly those that have been known for a relatively long time, have more than one name: A binary ionic compound is a. Why Is It Called A Binary Ionic Compound.
From www.showme.com
Describing Binary Ionic Compounds Science, Chemistry, Atoms, Elements Why Is It Called A Binary Ionic Compound Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. The metal cation is named first, followed by the nonmetal anion. A metal (which forms the cations) and a nonmetal (which forms the anions). Ions of main group elements were discussed thoroughly in. For example, nacl is a binary ionic compound. It is necessary, however,. Why Is It Called A Binary Ionic Compound.
From www.studypool.com
SOLUTION Binary ionic compounds Studypool Why Is It Called A Binary Ionic Compound The charge of the ions can be determined by their location within the periodic table. A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. The metal cation is named first, followed by the nonmetal anion. It is necessary, however, for a group 16 or 17. Binary ionic compounds are composed of. Why Is It Called A Binary Ionic Compound.
From saylordotorg.github.io
Naming Ionic Compounds Why Is It Called A Binary Ionic Compound Although a binary compound only contains two elements, it can contain. A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. In chemistry, a binary compound is a chemical compound consisting of exactly two different elements. It. Why Is It Called A Binary Ionic Compound.
From www.youtube.com
Naming Binary Ionic and Covalent Compounds YouTube Why Is It Called A Binary Ionic Compound Many compounds, particularly those that have been known for a relatively long time, have more than one name: Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. It is necessary, however, for a group 16 or 17. The charge of the ions can be determined by their location within the periodic table. A metal. Why Is It Called A Binary Ionic Compound.
From www.slideserve.com
PPT NAMING IONIC COMPOUNDS PowerPoint Presentation, free download Why Is It Called A Binary Ionic Compound Therefore, presence of a particular nonmetal does not guarantee that a binary compound is ionic. Many compounds, particularly those that have been known for a relatively long time, have more than one name: A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. It is necessary, however, for a group 16 or. Why Is It Called A Binary Ionic Compound.
From www.slideserve.com
PPT Nomenclature Binary Ionic Compounds PowerPoint Presentation Why Is It Called A Binary Ionic Compound Ions of main group elements were discussed thoroughly in. A metal (which forms the cations) and a nonmetal (which forms the anions). The metal cation is named first, followed by the nonmetal anion. It is necessary, however, for a group 16 or 17. For example, nacl is a binary ionic compound. Although a binary compound only contains two elements, it. Why Is It Called A Binary Ionic Compound.
From www.slideserve.com
PPT Binary Ionic Compounds PowerPoint Presentation, free download Why Is It Called A Binary Ionic Compound For example, nacl is a binary ionic compound. In chemistry, a binary compound is a chemical compound consisting of exactly two different elements. The metal cation is named first, followed by the nonmetal anion. A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. Many compounds, particularly those that have been known. Why Is It Called A Binary Ionic Compound.
From studylib.net
Formation of Binary Ionic Compounds Why Is It Called A Binary Ionic Compound The metal cation is named first, followed by the nonmetal anion. It is necessary, however, for a group 16 or 17. Ions of main group elements were discussed thoroughly in. A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. Although a binary compound only contains two elements, it can contain. The. Why Is It Called A Binary Ionic Compound.
From www.slideserve.com
PPT NAMING IONIC COMPOUNDS PowerPoint Presentation, free download Why Is It Called A Binary Ionic Compound For example, nacl is a binary ionic compound. A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. Many compounds, particularly those that have been known for a relatively long time, have more than one name: Ions of main group elements were discussed thoroughly in. It is necessary, however, for a group. Why Is It Called A Binary Ionic Compound.
From study.com
Chemical Formula for Ionic Compound Binary & Polyatomic Lesson Why Is It Called A Binary Ionic Compound It is necessary, however, for a group 16 or 17. For example, nacl is a binary ionic compound. Ions of main group elements were discussed thoroughly in. The metal cation is named first, followed by the nonmetal anion. In chemistry, a binary compound is a chemical compound consisting of exactly two different elements. The charge of the ions can be. Why Is It Called A Binary Ionic Compound.
From www.studypool.com
SOLUTION Binary ionic compounds Studypool Why Is It Called A Binary Ionic Compound A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. The charge of the ions can be determined by their location within the periodic table. Ions of main group elements were discussed thoroughly in. Although a binary compound only contains two elements, it can contain. For example, nacl is a binary ionic. Why Is It Called A Binary Ionic Compound.
From slidetodoc.com
Binary Ionic Compounds Formula to Name The Language Why Is It Called A Binary Ionic Compound Ions of main group elements were discussed thoroughly in. The metal cation is named first, followed by the nonmetal anion. It is necessary, however, for a group 16 or 17. Binary ionic compounds are composed of just two elements: A metal (which forms the cations) and a nonmetal (which forms the anions). A binary ionic compound is a compound composed. Why Is It Called A Binary Ionic Compound.
From studylib.net
Naming Binary Ionic Compounds Why Is It Called A Binary Ionic Compound For example, nacl is a binary ionic compound. Binary ionic compounds are composed of just two elements: In chemistry, a binary compound is a chemical compound consisting of exactly two different elements. Although a binary compound only contains two elements, it can contain. The charge of the ions can be determined by their location within the periodic table. A binary. Why Is It Called A Binary Ionic Compound.