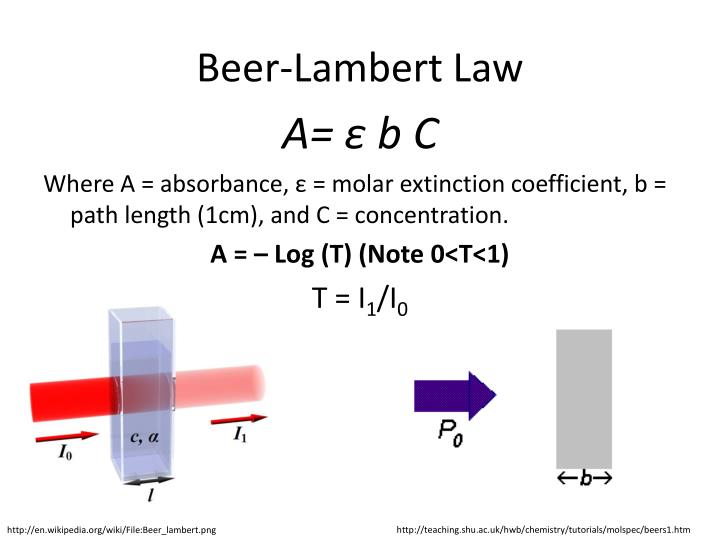
Beer's Law Terms . Beer's law is an equation that relates light's attenuation to a material's properties. The law states that a chemical's concentration is directly proportional to a solution's absorbance. There are two contributions to this fundamental. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law is a limiting law that is valid only for low concentrations of analyte. However, for simplicity’s sake, it is often simply called beer’s law. “the path length and concentration of a chemical are directly proportional to its absorption of light.” The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance.
from www.slideserve.com
Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. “the path length and concentration of a chemical are directly proportional to its absorption of light.” There are two contributions to this fundamental. However, for simplicity’s sake, it is often simply called beer’s law. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Beer's law is an equation that relates light's attenuation to a material's properties. Beer’s law is a limiting law that is valid only for low concentrations of analyte.
PPT Determining the Concentration of a Solution Beer’s Law
Beer's Law Terms Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer's law is an equation that relates light's attenuation to a material's properties. Beer’s law is a limiting law that is valid only for low concentrations of analyte. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. “the path length and concentration of a chemical are directly proportional to its absorption of light.” However, for simplicity’s sake, it is often simply called beer’s law. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. The law states that a chemical's concentration is directly proportional to a solution's absorbance. There are two contributions to this fundamental.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Terms Beer’s law is a limiting law that is valid only for low concentrations of analyte. “the path length and concentration of a chemical are directly proportional to its absorption of light.” Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The absorbance of a solution is directly proportional to the path length of. Beer's Law Terms.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Terms “the path length and concentration of a chemical are directly proportional to its absorption of light.” However, for simplicity’s sake, it is often simply called beer’s law. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. Since the concentration, path length and molar absorptivity are. Beer's Law Terms.
From www.youtube.com
Beer's Law Lab Procedure YouTube Beer's Law Terms The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. The law states that a chemical's concentration is directly proportional to a solution's absorbance. “the path length and concentration of a chemical are directly proportional to its absorption of light.” Beer lamberts law states a relationship. Beer's Law Terms.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law Terms Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. There are two contributions to this fundamental. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Beer lamberts law states a relationship between the attenuation of light through a substance. Beer's Law Terms.
From www.slideserve.com
PPT Atmospheric Transmission PowerPoint Presentation, free download Beer's Law Terms The law states that a chemical's concentration is directly proportional to a solution's absorbance. However, for simplicity’s sake, it is often simply called beer’s law. There are two contributions to this fundamental. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. You may use this. Beer's Law Terms.
From www.youtube.com
Analytical Instrumentation Tutorial 2 Beer Lambert Law YouTube Beer's Law Terms You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. There are two contributions to this fundamental. However, for simplicity’s sake, it is often simply called beer’s law. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the. Beer's Law Terms.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Terms However, for simplicity’s sake, it is often simply called beer’s law. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. “the path length and concentration of a chemical are directly proportional to its absorption of light.” Beer’s law is a limiting law that is valid only for low concentrations of. Beer's Law Terms.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer's Law Terms You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. There. Beer's Law Terms.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer's Law Terms “the path length and concentration of a chemical are directly proportional to its absorption of light.” The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's Law Terms.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer's Law Terms There are two contributions to this fundamental. Beer's law is an equation that relates light's attenuation to a material's properties. However, for simplicity’s sake, it is often simply called beer’s law. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer’s law is a limiting law that is valid only for low concentrations. Beer's Law Terms.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer's Law Terms “the path length and concentration of a chemical are directly proportional to its absorption of light.” You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.. Beer's Law Terms.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Terms Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer's law is an equation that relates light's attenuation to a material's properties. There are two contributions to this fundamental. The law states that. Beer's Law Terms.
From www.youtube.com
Beer's Law YouTube Beer's Law Terms Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer’s law is a limiting law that is valid only for low concentrations of analyte. There are two contributions. Beer's Law Terms.
From www.youtube.com
Derivation of Beer Lambert Law YouTube Beer's Law Terms However, for simplicity’s sake, it is often simply called beer’s law. There are two contributions to this fundamental. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. “the path length and concentration of a chemical are directly proportional to its absorption of light.” Beer's law is an equation that relates. Beer's Law Terms.
From www.slideserve.com
PPT LAB 3 PowerPoint Presentation, free download ID2225939 Beer's Law Terms The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. However, for simplicity’s sake, it is often simply called beer’s law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.. Beer's Law Terms.
From studylib.net
Beer`s Law Beer's Law Terms You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. There are two contributions to this fundamental. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's Law Terms.
From www.youtube.com
Ch 17 7 Beer's Law is Additive for All Components YouTube Beer's Law Terms You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species. Beer's Law Terms.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Terms There are two contributions to this fundamental. Beer's law is an equation that relates light's attenuation to a material's properties. “the path length and concentration of a chemical are directly proportional to its absorption of light.” Beer’s law is a limiting law that is valid only for low concentrations of analyte. You may use this relation to determine a chemical. Beer's Law Terms.
From www.youtube.com
BeerLambert law in easy way YouTube Beer's Law Terms Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. Beer’s law is a limiting law that is valid only for low concentrations of analyte. “the. Beer's Law Terms.
From lukewarmtakes.net
Spectrophotometry and Beer's Law Lukewarm Takes Beer's Law Terms Beer's law is an equation that relates light's attenuation to a material's properties. Beer’s law is a limiting law that is valid only for low concentrations of analyte. “the path length and concentration of a chemical are directly proportional to its absorption of light.” The law states that a chemical's concentration is directly proportional to a solution's absorbance. Beer’s law,. Beer's Law Terms.
From www.youtube.com
Beer Lambert law in Hindi Beer Lambert Law explained Lambert beer Beer's Law Terms The law states that a chemical's concentration is directly proportional to a solution's absorbance. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. There are two contributions to this fundamental. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the. Beer's Law Terms.
From www.youtube.com
Beer’s and Lamberts law B.Sc. Sem. 6 Physicssome important definitions Beer's Law Terms Beer's law is an equation that relates light's attenuation to a material's properties. However, for simplicity’s sake, it is often simply called beer’s law. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. The absorbance of a solution is directly proportional to the path length of the sample and. Beer's Law Terms.
From www.youtube.com
Spectrophotometric terms and BeerLambert Law YouTube Beer's Law Terms Beer’s law is a limiting law that is valid only for low concentrations of analyte. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. “the path length and concentration of a chemical are directly proportional to its absorption of light.” There are two contributions. Beer's Law Terms.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer's Law Terms Beer’s law is a limiting law that is valid only for low concentrations of analyte. “the path length and concentration of a chemical are directly proportional to its absorption of light.” Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy,. Beer's Law Terms.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Terms “the path length and concentration of a chemical are directly proportional to its absorption of light.” Beer’s law is a limiting law that is valid only for low concentrations of analyte. However, for simplicity’s sake, it is often simply called beer’s law. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or. Beer's Law Terms.
From www.youtube.com
Beer Law Lambert Law Limiting Law Absorbance Transmittance Beer's Law Terms Beer’s law is a limiting law that is valid only for low concentrations of analyte. Beer's law is an equation that relates light's attenuation to a material's properties. However, for simplicity’s sake, it is often simply called beer’s law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. Beer's Law Terms.
From www.youtube.com
Beer's Law Explanation and Example YouTube Beer's Law Terms However, for simplicity’s sake, it is often simply called beer’s law. The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The law states that a chemical's concentration is directly. Beer's Law Terms.
From www.slideshare.net
Beers law Beer's law Beer's Law Terms However, for simplicity’s sake, it is often simply called beer’s law. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. There are two contributions to this fundamental. Beer’s law is a limiting law that is. Beer's Law Terms.
From www.numerade.com
SOLVED Consider the Beer's Law equation A = εbc. Match the variable Beer's Law Terms You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. The law states that a chemical's concentration is directly proportional to a solution's absorbance. There are two contributions to this fundamental. Beer’s law is a limiting law that is valid only for low concentrations of analyte. However, for simplicity’s sake, it. Beer's Law Terms.
From www.youtube.com
Absorption law (ii) Beer’s Law UV and Visible Spectroscopy YouTube Beer's Law Terms However, for simplicity’s sake, it is often simply called beer’s law. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. “the path length and concentration of a chemical. Beer's Law Terms.
From www.youtube.com
Beer and Lambert Law Derivation YouTube Beer's Law Terms Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Beer's law is an equation that relates light's attenuation to a material's properties. There are two contributions to this fundamental. Beer’s law is a limiting law that is valid only. Beer's Law Terms.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Terms The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which. Beer's Law Terms.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer's Law Terms The absorbance of a solution is directly proportional to the path length of the sample and concentration of the attenuation species in the solution. Beer's law is an equation that relates light's attenuation to a material's properties. However, for simplicity’s sake, it is often simply called beer’s law. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy. Beer's Law Terms.
From www.slideserve.com
PPT Beers Law for a Single Component Sample PowerPoint Presentation Beer's Law Terms The law states that a chemical's concentration is directly proportional to a solution's absorbance. Beer’s law is a limiting law that is valid only for low concentrations of analyte. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You may use this relation to. Beer's Law Terms.
From www.youtube.com
Beer's Law Concept, Calculations, & Lab YouTube Beer's Law Terms You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. “the path length and concentration of a chemical are directly proportional. Beer's Law Terms.