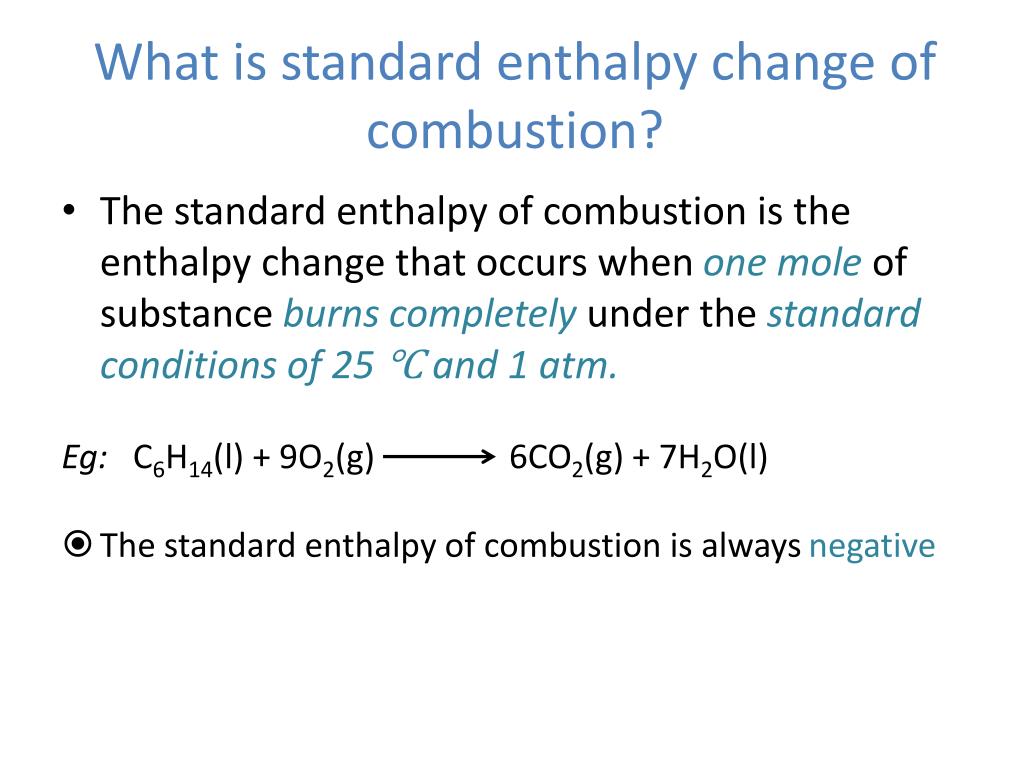
Standard Enthalpy Change Of Formation Unit . Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. Most are defined in kilojoules per. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. Standard conditions (298k and 100kpa) are used and reactants must be in their standard states (usual state at standard conditions). The ⦵ symbol is used to represent standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy change of formation is measured in units of energy per amount of substance.
from www.slideserve.com
The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. Most are defined in kilojoules per. The ⦵ symbol is used to represent standard. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. The standard enthalpy change of formation is measured in units of energy per amount of substance. Standard conditions (298k and 100kpa) are used and reactants must be in their standard states (usual state at standard conditions).
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint
Standard Enthalpy Change Of Formation Unit Most are defined in kilojoules per. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. The standard enthalpy change of formation is measured in units of energy per amount of substance. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. Most are defined in kilojoules per. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard conditions (298k and 100kpa) are used and reactants must be in their standard states (usual state at standard conditions). The ⦵ symbol is used to represent standard.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Change Of Formation Unit Standard conditions (298k and 100kpa) are used and reactants must be in their standard states (usual state at standard conditions). The ⦵ symbol is used to represent standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Most are defined in kilojoules per. The standard enthalpy. Standard Enthalpy Change Of Formation Unit.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Enthalpy Change Of Formation Unit Most are defined in kilojoules per. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. Standard conditions (298k and 100kpa) are used and reactants must be in their standard states (usual state at standard conditions). The standard enthalpy change of formation is measured in units of energy. Standard Enthalpy Change Of Formation Unit.
From www.vrogue.co
Ppt Enthalpy Of Formation Combustion Of Methanol Powe vrogue.co Standard Enthalpy Change Of Formation Unit The ⦵ symbol is used to represent standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. Standard conditions (298k and 100kpa). Standard Enthalpy Change Of Formation Unit.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Enthalpy Change Of Formation Unit Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. The ⦵ symbol is used to represent standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a. Standard Enthalpy Change Of Formation Unit.
From www.slideserve.com
PPT Energetics PowerPoint Presentation ID1204656 Standard Enthalpy Change Of Formation Unit Standard conditions (298k and 100kpa) are used and reactants must be in their standard states (usual state at standard conditions). Most are defined in kilojoules per. The ⦵ symbol is used to represent standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy. Standard Enthalpy Change Of Formation Unit.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5773812 Standard Enthalpy Change Of Formation Unit The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. Standard enthalpy of formation is the enthalpy change when one mole of. Standard Enthalpy Change Of Formation Unit.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Change Of Formation Unit Standard conditions (298k and 100kpa) are used and reactants must be in their standard states (usual state at standard conditions). The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy change of formation is measured in units of energy per amount of substance.. Standard Enthalpy Change Of Formation Unit.
From www.thesciencehive.co.uk
Enthalpy Changes — the science hive Standard Enthalpy Change Of Formation Unit Standard conditions (298k and 100kpa) are used and reactants must be in their standard states (usual state at standard conditions). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from. Standard Enthalpy Change Of Formation Unit.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Change Of Formation Unit Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy change of formation is measured in units of energy. Standard Enthalpy Change Of Formation Unit.
From www.youtube.com
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL) YouTube Standard Enthalpy Change Of Formation Unit Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. Most are defined in kilojoules per. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The ⦵ symbol is used to represent standard. The. Standard Enthalpy Change Of Formation Unit.
From www.hotzxgirl.com
Ppt Example Using Enthalpy Of Combustion And Formation Powerpoint Hot Standard Enthalpy Change Of Formation Unit Most are defined in kilojoules per. The standard enthalpy change of formation is measured in units of energy per amount of substance. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. The ⦵ symbol is used to represent standard. 193 rows in chemistry and thermodynamics,. Standard Enthalpy Change Of Formation Unit.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Change Of Formation Unit The standard enthalpy change of formation is measured in units of energy per amount of substance. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its. Standard Enthalpy Change Of Formation Unit.
From chemistry.stackexchange.com
thermodynamics Calculating Enthalpy of formation versus Calculating Standard Enthalpy Change Of Formation Unit The standard enthalpy change of formation is measured in units of energy per amount of substance. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The ⦵ symbol is used to represent standard. Most are defined in kilojoules per. Standard conditions (298k and 100kpa) are. Standard Enthalpy Change Of Formation Unit.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Change Of Formation Unit Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. The standard enthalpy change of formation is measured in units of energy per amount of substance. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance. Standard Enthalpy Change Of Formation Unit.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Enthalpy Change Of Formation Unit The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Enthalpy Change Of Formation Unit.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Change Of Formation Unit Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or. Standard Enthalpy Change Of Formation Unit.
From www.bartleby.com
Answered Using the standard enthalpies of… bartleby Standard Enthalpy Change Of Formation Unit The standard enthalpy change of formation is measured in units of energy per amount of substance. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. The ⦵ symbol is used to represent standard. Standard conditions (298k and 100kpa) are used and reactants must be in. Standard Enthalpy Change Of Formation Unit.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Change Of Formation Unit The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The ⦵ symbol is used to represent standard. Most are defined in kilojoules per. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements,. Standard Enthalpy Change Of Formation Unit.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Change Of Formation Unit The standard enthalpy change of formation is measured in units of energy per amount of substance. Most are defined in kilojoules per. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. Standard enthalpy of formation is the enthalpy change when one mole of any compound. Standard Enthalpy Change Of Formation Unit.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Change Of Formation Unit Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. The standard enthalpy change of formation is measured in units of energy per amount. Standard Enthalpy Change Of Formation Unit.
From exosbjzsg.blob.core.windows.net
Standard Enthalpy Of Formation Al2O3 at Lois Woodburn blog Standard Enthalpy Change Of Formation Unit The ⦵ symbol is used to represent standard. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy of formation. Standard Enthalpy Change Of Formation Unit.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free Standard Enthalpy Change Of Formation Unit The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. Standard conditions (298k and 100kpa) are used and reactants must be in their standard states (usual state at standard conditions). Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed. Standard Enthalpy Change Of Formation Unit.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Change Of Formation Unit Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. Most are defined in kilojoules per. 193 rows in chemistry and thermodynamics,. Standard Enthalpy Change Of Formation Unit.
From mungfali.com
Enthalpy Change Of Formation Equation Standard Enthalpy Change Of Formation Unit Standard conditions (298k and 100kpa) are used and reactants must be in their standard states (usual state at standard conditions). The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Enthalpy Change Of Formation Unit.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpy Change Of Formation Unit Most are defined in kilojoules per. Standard conditions (298k and 100kpa) are used and reactants must be in their standard states (usual state at standard conditions). Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. 193 rows in chemistry and thermodynamics, the standard enthalpy of. Standard Enthalpy Change Of Formation Unit.
From www.chegg.com
Solved Use The Standard Enthalpies Of Formation In The Ta... Standard Enthalpy Change Of Formation Unit Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. Standard conditions (298k and 100kpa) are used and reactants must be in their standard states (usual state at standard conditions). The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree. Standard Enthalpy Change Of Formation Unit.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Standard Enthalpy Change Of Formation Unit The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The ⦵ symbol is used to represent standard. Enthalpy of formation (\. Standard Enthalpy Change Of Formation Unit.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Enthalpy Change Of Formation Unit The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Most are defined in kilojoules per. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. Standard enthalpy of formation is the. Standard Enthalpy Change Of Formation Unit.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Change Of Formation Unit The ⦵ symbol is used to represent standard. The standard enthalpy change of formation is measured in units of energy per amount of substance. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. Standard enthalpy of formation is the enthalpy change when one mole of. Standard Enthalpy Change Of Formation Unit.
From www.slideserve.com
PPT Energetics PowerPoint Presentation, free download ID3196359 Standard Enthalpy Change Of Formation Unit The ⦵ symbol is used to represent standard. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. Most are defined in kilojoules per.. Standard Enthalpy Change Of Formation Unit.
From www.youtube.com
R1.2.3 / R1.2.4 Standard enthalpy change of combustion (HL) YouTube Standard Enthalpy Change Of Formation Unit The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of. Standard Enthalpy Change Of Formation Unit.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Change Of Formation Unit Standard conditions (298k and 100kpa) are used and reactants must be in their standard states (usual state at standard conditions). The ⦵ symbol is used to represent standard. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. 193 rows in chemistry and thermodynamics, the standard enthalpy of. Standard Enthalpy Change Of Formation Unit.
From srkvlvhthzubm.blogspot.com
How To Calculate Standard Enthalpy Change Of Formation For most Standard Enthalpy Change Of Formation Unit Most are defined in kilojoules per. The standard enthalpy change of formation is measured in units of energy per amount of substance. Enthalpy of formation (\ (δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. The standard enthalpy of formation is a measure of the energy released or consumed. Standard Enthalpy Change Of Formation Unit.
From hotcore.info
Enthalpy Change Standard Enthalpy Change Of Formation Unit Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. The ⦵ symbol is used to represent standard. The standard enthalpy of formation is. Standard Enthalpy Change Of Formation Unit.
From www.vrogue.co
Standard Enthalpy Of Reaction Units Slide Share vrogue.co Standard Enthalpy Change Of Formation Unit Most are defined in kilojoules per. The standard enthalpy change of formation is measured in units of energy per amount of substance. Standard conditions (298k and 100kpa) are used and reactants must be in their standard states (usual state at standard conditions). The ⦵ symbol is used to represent standard. Standard enthalpy of formation is the enthalpy change when one. Standard Enthalpy Change Of Formation Unit.