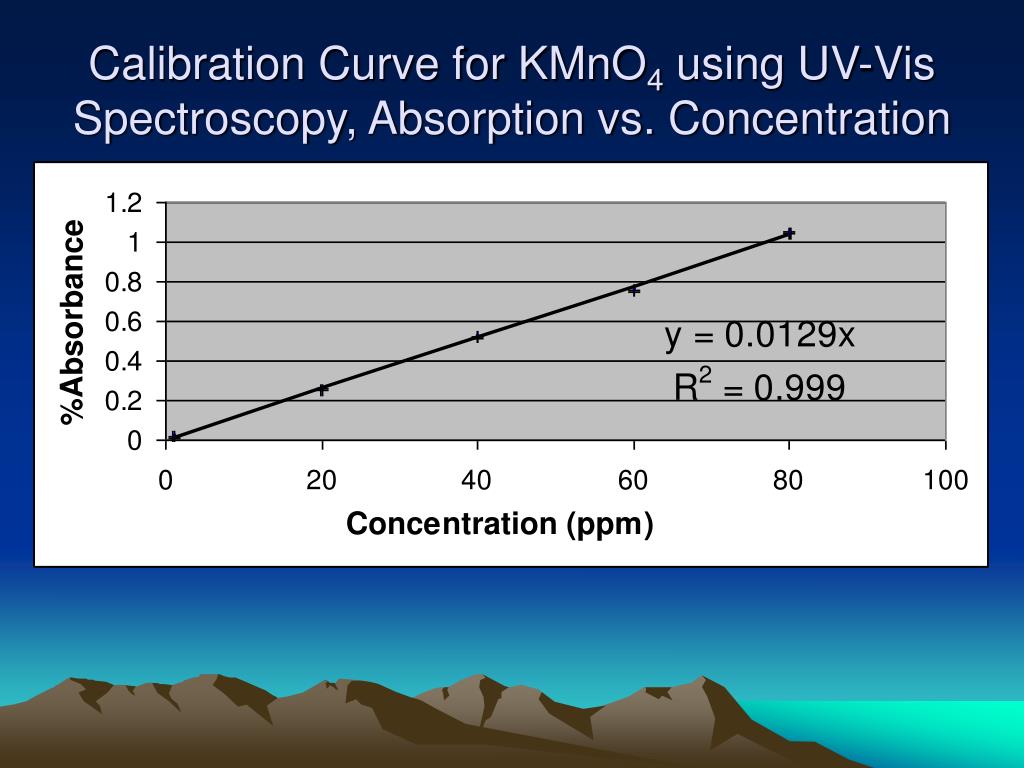
Calibration Curve Absorbance Vs Concentration . Concentration is the amount of a substance present in a specific volume of a solution. It calculates the concentration of the unknown. Linear regression of straight line calibration curves. The difference between calibration curve absorbance and concentration is their nature. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. To make a calibration curve, the value for the absorbances of each of the spectral curves at the highest absorbing wavelength, is plotted in a graph similar to that in. Use compound z to prepare a set of calibration solutions, construct a calibration curve and then use the curve to predict the concentration of z in solution s1. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following.
from www.slideserve.com
The difference between calibration curve absorbance and concentration is their nature. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. To make a calibration curve, the value for the absorbances of each of the spectral curves at the highest absorbing wavelength, is plotted in a graph similar to that in. Concentration is the amount of a substance present in a specific volume of a solution. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Linear regression of straight line calibration curves. Use compound z to prepare a set of calibration solutions, construct a calibration curve and then use the curve to predict the concentration of z in solution s1. It calculates the concentration of the unknown.
PPT Ultraviolet/Visible (UVVis) Spectroscopy of Potassium
Calibration Curve Absorbance Vs Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Use compound z to prepare a set of calibration solutions, construct a calibration curve and then use the curve to predict the concentration of z in solution s1. Linear regression of straight line calibration curves. To make a calibration curve, the value for the absorbances of each of the spectral curves at the highest absorbing wavelength, is plotted in a graph similar to that in. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Concentration is the amount of a substance present in a specific volume of a solution. It calculates the concentration of the unknown. The difference between calibration curve absorbance and concentration is their nature.
From www.slideserve.com
PPT Ultraviolet/Visible (UVVis) Spectroscopy of Potassium Calibration Curve Absorbance Vs Concentration A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Linear regression of straight line calibration curves. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Use compound z to prepare a set of calibration solutions, construct a calibration. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Absorbance vs. concentration calibration curve Download Scientific Calibration Curve Absorbance Vs Concentration To make a calibration curve, the value for the absorbances of each of the spectral curves at the highest absorbing wavelength, is plotted in a graph similar to that in. The difference between calibration curve absorbance and concentration is their nature. Concentration is the amount of a substance present in a specific volume of a solution. A calibration curve, also. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Calibration curve representing the absorbance vs. the gaseous toluene Calibration Curve Absorbance Vs Concentration Linear regression of straight line calibration curves. It calculates the concentration of the unknown. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Use compound z to prepare a. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Absorbance spectrum (a), and final calibration curve (b) for Nile red Calibration Curve Absorbance Vs Concentration A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Linear regression of straight line calibration curves. The difference between calibration curve absorbance and concentration is their nature. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. It calculates. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Calibration curve for absorbance versus concentration of gentamicin Calibration Curve Absorbance Vs Concentration Concentration is the amount of a substance present in a specific volume of a solution. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The difference between calibration curve absorbance and concentration is their nature. It calculates the concentration of the unknown. Linear regression of straight line calibration curves.. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
(a) Calibration curve of absorbance versus BSA concentrations, (b Calibration Curve Absorbance Vs Concentration It calculates the concentration of the unknown. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Use compound z to prepare a set of calibration solutions, construct a calibration curve and then use the curve to predict the concentration of z in solution s1. To make a calibration curve,. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Calibration curve for catechin concentration vs. absorbance. Download Calibration Curve Absorbance Vs Concentration The difference between calibration curve absorbance and concentration is their nature. To make a calibration curve, the value for the absorbances of each of the spectral curves at the highest absorbing wavelength, is plotted in a graph similar to that in. Use compound z to prepare a set of calibration solutions, construct a calibration curve and then use the curve. Calibration Curve Absorbance Vs Concentration.
From cscdb.nku.edu
Spectrophotometry & Dilutions Calibration Curve Absorbance Vs Concentration It calculates the concentration of the unknown. Linear regression of straight line calibration curves. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. To make a calibration curve, the value for the absorbances of each of the spectral curves at the highest absorbing wavelength, is plotted in a graph. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Calibration curve for curcumin, obtained by plotting the absorbance of Calibration Curve Absorbance Vs Concentration The difference between calibration curve absorbance and concentration is their nature. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. To make a calibration curve, the value for the absorbances of each of the spectral curves at the highest absorbing wavelength, is plotted in a graph similar to that. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Standard calibration curve of Dglucose (absorbance measured at 489 nm Calibration Curve Absorbance Vs Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Linear regression of straight line calibration curves. It calculates the concentration of the unknown. To make a calibration curve, the. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Calibration curve and absorption spectra for various concentrations of Calibration Curve Absorbance Vs Concentration It calculates the concentration of the unknown. Use compound z to prepare a set of calibration solutions, construct a calibration curve and then use the curve to predict the concentration of z in solution s1. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Concentration is the amount of. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Calibration curve of crotonic acid concentration versus absorbance at Calibration Curve Absorbance Vs Concentration Linear regression of straight line calibration curves. The difference between calibration curve absorbance and concentration is their nature. Concentration is the amount of a substance present in a specific volume of a solution. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Since the concentration, path length and molar. Calibration Curve Absorbance Vs Concentration.
From pediaa.com
What is the Difference Between Calibration Curve Absorbance and Calibration Curve Absorbance Vs Concentration To make a calibration curve, the value for the absorbances of each of the spectral curves at the highest absorbing wavelength, is plotted in a graph similar to that in. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Use compound z to prepare a set of calibration solutions,. Calibration Curve Absorbance Vs Concentration.
From www.atozcolor.com
How to Make a Calibration Curve in Excel A to Z Color Calibration Curve Absorbance Vs Concentration A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. To make a calibration curve, the value for the absorbances of each of the spectral curves at the highest absorbing. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Calibration curve of absorbance versus concentration. Download Calibration Curve Absorbance Vs Concentration To make a calibration curve, the value for the absorbances of each of the spectral curves at the highest absorbing wavelength, is plotted in a graph similar to that in. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Use compound z to prepare a set of calibration solutions,. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Calibration curve for copper(II) concentration vs absorbance Calibration Curve Absorbance Vs Concentration Use compound z to prepare a set of calibration solutions, construct a calibration curve and then use the curve to predict the concentration of z in solution s1. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The difference between calibration curve absorbance and concentration is their nature. Concentration. Calibration Curve Absorbance Vs Concentration.
From inside107and109.blogspot.co.uk
inside 107 and 109 November 2016 Calibration Curve Absorbance Vs Concentration To make a calibration curve, the value for the absorbances of each of the spectral curves at the highest absorbing wavelength, is plotted in a graph similar to that in. Concentration is the amount of a substance present in a specific volume of a solution. Linear regression of straight line calibration curves. Since the concentration, path length and molar absorptivity. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Calibration curves (absorbance vs. concentration) with Fe(III Calibration Curve Absorbance Vs Concentration To make a calibration curve, the value for the absorbances of each of the spectral curves at the highest absorbing wavelength, is plotted in a graph similar to that in. The difference between calibration curve absorbance and concentration is their nature. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Calibration curve of light absorbance against methyl violet Calibration Curve Absorbance Vs Concentration It calculates the concentration of the unknown. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Linear regression of straight line calibration curves. To make a calibration curve, the value for the absorbances of each of the spectral curves at the highest absorbing wavelength, is plotted in a graph. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
(a) concentration vs. density calibration curve, (b) solubility curves Calibration Curve Absorbance Vs Concentration Use compound z to prepare a set of calibration solutions, construct a calibration curve and then use the curve to predict the concentration of z in solution s1. It calculates the concentration of the unknown. To make a calibration curve, the value for the absorbances of each of the spectral curves at the highest absorbing wavelength, is plotted in a. Calibration Curve Absorbance Vs Concentration.
From www.slideserve.com
PPT Experiment 22 Colorimetric determination of an equilibrium Calibration Curve Absorbance Vs Concentration It calculates the concentration of the unknown. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Concentration is the amount of a substance present in a specific volume of a solution. Linear regression of straight line calibration curves. Use compound z to prepare a set of calibration solutions, construct. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Absorbance calibration curves. Download Scientific Diagram Calibration Curve Absorbance Vs Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Use compound z to prepare a set of calibration solutions, construct a calibration curve and then use the curve to predict the concentration of z in solution s1. The difference between calibration curve absorbance and concentration is their nature. Concentration. Calibration Curve Absorbance Vs Concentration.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Calibration Curve Absorbance Vs Concentration Concentration is the amount of a substance present in a specific volume of a solution. To make a calibration curve, the value for the absorbances of each of the spectral curves at the highest absorbing wavelength, is plotted in a graph similar to that in. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Calibration Curve Absorbance Vs Concentration.
From mavink.com
Absorbance Calibration Curve Calibration Curve Absorbance Vs Concentration A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. It calculates the concentration of the unknown. Concentration is the amount of a substance present in a specific volume of. Calibration Curve Absorbance Vs Concentration.
From www.mdpi.com
Materials Free FullText EnergyDependent Particle Size Calibration Curve Absorbance Vs Concentration A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Concentration is the amount of a substance present in a specific volume of a solution. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. To make a calibration curve,. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Calibration curve of AsAc concentration (ppm) vs absorbance Download Calibration Curve Absorbance Vs Concentration A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. It calculates the concentration of the unknown. Use compound z to prepare a set of calibration solutions, construct a calibration. Calibration Curve Absorbance Vs Concentration.
From mavink.com
Absorbance Vs. Concentration Graph Calibration Curve Absorbance Vs Concentration Linear regression of straight line calibration curves. Use compound z to prepare a set of calibration solutions, construct a calibration curve and then use the curve to predict the concentration of z in solution s1. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. It calculates the concentration of. Calibration Curve Absorbance Vs Concentration.
From mungfali.com
Standard Curve Graph Calibration Curve Absorbance Vs Concentration The difference between calibration curve absorbance and concentration is their nature. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. Use compound z to prepare a set of calibration solutions, construct a calibration curve and then use the curve to predict the concentration of z in solution s1. Concentration. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Absorbance vs. concentration calibration curve Download Scientific Calibration Curve Absorbance Vs Concentration Concentration is the amount of a substance present in a specific volume of a solution. The difference between calibration curve absorbance and concentration is their nature. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. A calibration curve, also known as a standard curve, is a way to identify. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Calibration curve of absorbance versus concentration of gallic acid [mg Calibration Curve Absorbance Vs Concentration Linear regression of straight line calibration curves. The difference between calibration curve absorbance and concentration is their nature. Use compound z to prepare a set of calibration solutions, construct a calibration curve and then use the curve to predict the concentration of z in solution s1. To make a calibration curve, the value for the absorbances of each of the. Calibration Curve Absorbance Vs Concentration.
From chem-net.blogspot.com
Quantitative Analysis by Atomic Absorption Chemistry Net Calibration Curve Absorbance Vs Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Concentration is the amount of a substance present in a specific volume of a solution. The difference between calibration curve absorbance and concentration is their nature. A calibration curve, also known as a standard curve, is a way to identify. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Calibration curve (concentration vs absorbance) for Ni, Cr, Cu, Mn, Co Calibration Curve Absorbance Vs Concentration Concentration is the amount of a substance present in a specific volume of a solution. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. It calculates the concentration of the unknown. Linear regression of straight line calibration curves. Since the concentration, path length and molar absorptivity are all directly. Calibration Curve Absorbance Vs Concentration.
From chem.libretexts.org
4.4 UVVisible Spectroscopy Chemistry LibreTexts Calibration Curve Absorbance Vs Concentration Linear regression of straight line calibration curves. Concentration is the amount of a substance present in a specific volume of a solution. Use compound z to prepare a set of calibration solutions, construct a calibration curve and then use the curve to predict the concentration of z in solution s1. It calculates the concentration of the unknown. A calibration curve,. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Calibration curve of absorbance versus concentration. Download Calibration Curve Absorbance Vs Concentration Concentration is the amount of a substance present in a specific volume of a solution. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. It calculates the concentration of the unknown. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Calibration Curve Absorbance Vs Concentration.
From www.researchgate.net
Graph of Absorbance vs Concentration Download Scientific Diagram Calibration Curve Absorbance Vs Concentration Use compound z to prepare a set of calibration solutions, construct a calibration curve and then use the curve to predict the concentration of z in solution s1. It calculates the concentration of the unknown. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. The difference between calibration curve. Calibration Curve Absorbance Vs Concentration.