In Galvanic Current The Positive Electrode Is . This means that if the electrons. Why cathode is positive in the galvanic cell? The pt electrode in the permanganate solution is the cathode; In a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently. It is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs. Conventional current flow is from positive to negative, which is opposite to the direction of the electron flow. The one in the tin solution is the anode. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. This seems reasonable as the anode is the source of. The positive electrode, on the other hand,. The anode is the electrode where oxidation (loss of electrons) occurs; In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode.
from classnotes.org.in
Conventional current flow is from positive to negative, which is opposite to the direction of the electron flow. The pt electrode in the permanganate solution is the cathode; In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. This seems reasonable as the anode is the source of. The anode is the electrode where oxidation (loss of electrons) occurs; It is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs. The positive electrode, on the other hand,. Why cathode is positive in the galvanic cell? In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. In a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently.
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12
In Galvanic Current The Positive Electrode Is The anode is the electrode where oxidation (loss of electrons) occurs; In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. In a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. Why cathode is positive in the galvanic cell? By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag. It is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs. The pt electrode in the permanganate solution is the cathode; This seems reasonable as the anode is the source of. The one in the tin solution is the anode. This means that if the electrons. The positive electrode, on the other hand,. Conventional current flow is from positive to negative, which is opposite to the direction of the electron flow. The anode is the electrode where oxidation (loss of electrons) occurs;
From byjus.com
In electrolysis what are the positive and negative electrodes known as?? In Galvanic Current The Positive Electrode Is The positive electrode, on the other hand,. Why cathode is positive in the galvanic cell? By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag. The one in the tin solution is the anode. This means that if. In Galvanic Current The Positive Electrode Is.
From dxoufitvg.blob.core.windows.net
What's The Difference Between Galvanic And Electrochemical Cells at In Galvanic Current The Positive Electrode Is By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag. This means that if the electrons. The positive electrode, on the other hand,. The anode is the electrode where oxidation (loss of electrons) occurs; The one in the. In Galvanic Current The Positive Electrode Is.
From courses.lumenlearning.com
Galvanic Cells Chemistry In Galvanic Current The Positive Electrode Is It is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs. The one in the tin solution is the anode. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag.. In Galvanic Current The Positive Electrode Is.
From chemistry.stackexchange.com
electrochemistry Standard electrode potential for electrolytic vs In Galvanic Current The Positive Electrode Is Why cathode is positive in the galvanic cell? In a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently. It is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs. In any electrochemical cell (electrolytic or galvanic) the electrode at which. In Galvanic Current The Positive Electrode Is.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Copper In Galvanic Current The Positive Electrode Is The pt electrode in the permanganate solution is the cathode; This means that if the electrons. This seems reasonable as the anode is the source of. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. It is the negative electrode in a galvanic cell since electrons are left on the electrode when. In Galvanic Current The Positive Electrode Is.
From glossary.periodni.com
Galvanic Chemistry Dictionary & Glossary In Galvanic Current The Positive Electrode Is By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. This means that if the electrons. In a galvanic cell, it. In Galvanic Current The Positive Electrode Is.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps In Galvanic Current The Positive Electrode Is The anode is the electrode where oxidation (loss of electrons) occurs; This seems reasonable as the anode is the source of. The pt electrode in the permanganate solution is the cathode; The positive electrode, on the other hand,. The one in the tin solution is the anode. This means that if the electrons. Why cathode is positive in the galvanic. In Galvanic Current The Positive Electrode Is.
From kladwjlgf.blob.core.windows.net
Electrochemistry Galvanic Cells at Roberto Alvarado blog In Galvanic Current The Positive Electrode Is This means that if the electrons. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The anode is the electrode where oxidation (loss of electrons) occurs; The pt electrode in the permanganate solution is the cathode; Conventional current flow is from positive to negative, which is opposite to the direction of the. In Galvanic Current The Positive Electrode Is.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working In Galvanic Current The Positive Electrode Is The positive electrode, on the other hand,. Why cathode is positive in the galvanic cell? Conventional current flow is from positive to negative, which is opposite to the direction of the electron flow. This means that if the electrons. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil). In Galvanic Current The Positive Electrode Is.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode In Galvanic Current The Positive Electrode Is The pt electrode in the permanganate solution is the cathode; This seems reasonable as the anode is the source of. Why cathode is positive in the galvanic cell? It is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs. The one in the tin solution is the anode. Conventional current flow is. In Galvanic Current The Positive Electrode Is.
From brahmavad.in
Current Electricity Swami Vivekananda In Galvanic Current The Positive Electrode Is By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. It is the negative electrode in a galvanic cell since electrons. In Galvanic Current The Positive Electrode Is.
From stock.adobe.com
Vector scientific illustration of the electrolysis processes. Set of In Galvanic Current The Positive Electrode Is The pt electrode in the permanganate solution is the cathode; Why cathode is positive in the galvanic cell? The positive electrode, on the other hand,. The one in the tin solution is the anode. In a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently. It is the negative. In Galvanic Current The Positive Electrode Is.
From www.slideserve.com
PPT Oxidation PowerPoint Presentation, free download ID4827900 In Galvanic Current The Positive Electrode Is This seems reasonable as the anode is the source of. The positive electrode, on the other hand,. The pt electrode in the permanganate solution is the cathode; This means that if the electrons. It is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs. The one in the tin solution is the. In Galvanic Current The Positive Electrode Is.
From fixlibrarybaladinas3c.z4.web.core.windows.net
What Happens At The Cathode In Electrolysis In Galvanic Current The Positive Electrode Is It is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs. This seems reasonable as the anode is the source of. Why cathode is positive in the galvanic cell? In a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently. The. In Galvanic Current The Positive Electrode Is.
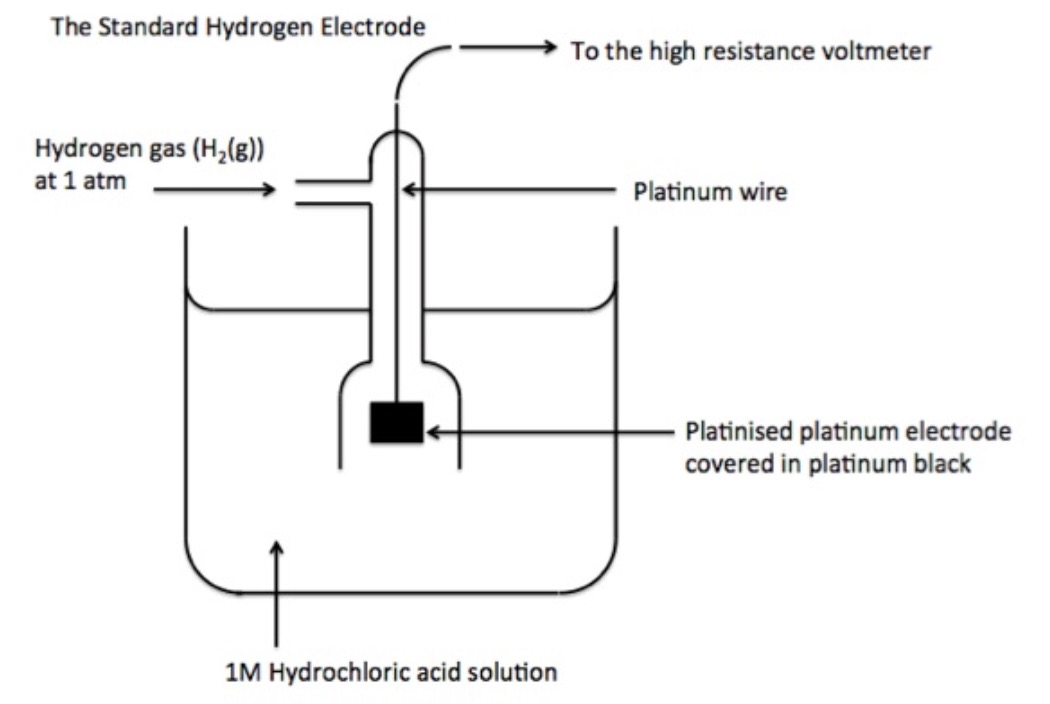
From loeduhpag.blob.core.windows.net
Hydrogen Electrode Galvanic Cell at Justin Mclean blog In Galvanic Current The Positive Electrode Is The positive electrode, on the other hand,. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Why cathode is positive in the galvanic cell? The pt electrode in the permanganate solution is the cathode; In a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from. In Galvanic Current The Positive Electrode Is.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download In Galvanic Current The Positive Electrode Is By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag. The anode is the electrode where oxidation (loss of electrons) occurs; In a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons. In Galvanic Current The Positive Electrode Is.
From www.numerade.com
SOLVEDIdentify the components of the galvanic electrochemical cell In Galvanic Current The Positive Electrode Is This means that if the electrons. It is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs. This seems reasonable as the anode is the source of. Conventional current flow is from positive to negative, which is opposite to the direction of the electron flow. By definition, the anode of an electrochemical. In Galvanic Current The Positive Electrode Is.
From www.youtube.com
Galvanic Cell Definition, Construction, Working, Example, Diagram In Galvanic Current The Positive Electrode Is In a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently. The pt electrode in the permanganate solution is the cathode; The anode is the electrode where oxidation (loss of electrons) occurs; By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this. In Galvanic Current The Positive Electrode Is.
From www.nagwa.com
Question Video Identifying the Positive Electrode of a Galvanic Cell In Galvanic Current The Positive Electrode Is In a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag. The anode is the electrode where. In Galvanic Current The Positive Electrode Is.
From www.youtube.com
Calculating the Cell Potential of Electrochemical Cells. (Adv Chem Ch In Galvanic Current The Positive Electrode Is The one in the tin solution is the anode. The positive electrode, on the other hand,. This seems reasonable as the anode is the source of. This means that if the electrons. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. It is the negative electrode in a galvanic cell since electrons. In Galvanic Current The Positive Electrode Is.
From dxotvmkyy.blob.core.windows.net
Galvanic Current Positive Negative at Anita Reilly blog In Galvanic Current The Positive Electrode Is By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag. The pt electrode in the permanganate solution is the cathode; This means that if the electrons. In a galvanic (voltaic) cell, the anode is considered negative and the. In Galvanic Current The Positive Electrode Is.
From 2012books.lardbucket.org
Standard Potentials In Galvanic Current The Positive Electrode Is Why cathode is positive in the galvanic cell? Conventional current flow is from positive to negative, which is opposite to the direction of the electron flow. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag. It is. In Galvanic Current The Positive Electrode Is.
From www.bigstockphoto.com
Galvanic Cell Simple & Easy Image & Photo Bigstock In Galvanic Current The Positive Electrode Is The pt electrode in the permanganate solution is the cathode; The positive electrode, on the other hand,. Why cathode is positive in the galvanic cell? In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. It is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs.. In Galvanic Current The Positive Electrode Is.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE In Galvanic Current The Positive Electrode Is Conventional current flow is from positive to negative, which is opposite to the direction of the electron flow. It is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs. In a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently. In. In Galvanic Current The Positive Electrode Is.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts In Galvanic Current The Positive Electrode Is Conventional current flow is from positive to negative, which is opposite to the direction of the electron flow. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag. In a galvanic cell, it acts as the positive electrode. In Galvanic Current The Positive Electrode Is.
From es.vecteezy.com
celda electroquímica o celda galvánica, la celda daniell 18989226 In Galvanic Current The Positive Electrode Is Conventional current flow is from positive to negative, which is opposite to the direction of the electron flow. It is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs. In a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently. The. In Galvanic Current The Positive Electrode Is.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General In Galvanic Current The Positive Electrode Is This seems reasonable as the anode is the source of. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. It is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs. Why cathode is positive in the galvanic cell? The one in the tin solution is. In Galvanic Current The Positive Electrode Is.
From www.thoughtco.com
How to Define Anode and Cathode In Galvanic Current The Positive Electrode Is By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag. The one in the tin solution is the anode. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. The pt electrode. In Galvanic Current The Positive Electrode Is.
From www.coursehero.com
[Solved] draw a galvanic cell and label the anode/cathode, cell In Galvanic Current The Positive Electrode Is This means that if the electrons. The positive electrode, on the other hand,. Conventional current flow is from positive to negative, which is opposite to the direction of the electron flow. The one in the tin solution is the anode. It is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs. This. In Galvanic Current The Positive Electrode Is.
From courses.lumenlearning.com
Electrolysis Chemistry In Galvanic Current The Positive Electrode Is In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The anode is the electrode where oxidation (loss of electrons) occurs; Why cathode is positive in the galvanic cell? It is the negative electrode in a galvanic cell since electrons are left on the electrode when oxidation occurs. Conventional current flow is from. In Galvanic Current The Positive Electrode Is.
From slideplayer.com
Electric Current Chapter ppt download In Galvanic Current The Positive Electrode Is The pt electrode in the permanganate solution is the cathode; In a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently. Why cathode is positive in the galvanic cell? The one in the tin solution is the anode. In any electrochemical cell (electrolytic or galvanic) the electrode at which. In Galvanic Current The Positive Electrode Is.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 In Galvanic Current The Positive Electrode Is Why cathode is positive in the galvanic cell? The pt electrode in the permanganate solution is the cathode; The one in the tin solution is the anode. The positive electrode, on the other hand,. In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. In a galvanic cell, it acts as the positive. In Galvanic Current The Positive Electrode Is.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an In Galvanic Current The Positive Electrode Is The one in the tin solution is the anode. The anode is the electrode where oxidation (loss of electrons) occurs; In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. This seems reasonable as the anode is the source of. Why cathode is positive in the galvanic cell? In a galvanic cell, it acts. In Galvanic Current The Positive Electrode Is.
From slideplayer.com
Electrochemistry Chapter ppt download In Galvanic Current The Positive Electrode Is The anode is the electrode where oxidation (loss of electrons) occurs; In a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently. The pt electrode in the permanganate solution is the cathode; By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this. In Galvanic Current The Positive Electrode Is.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and In Galvanic Current The Positive Electrode Is The one in the tin solution is the anode. The pt electrode in the permanganate solution is the cathode; By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag. The anode is the electrode where oxidation (loss of. In Galvanic Current The Positive Electrode Is.