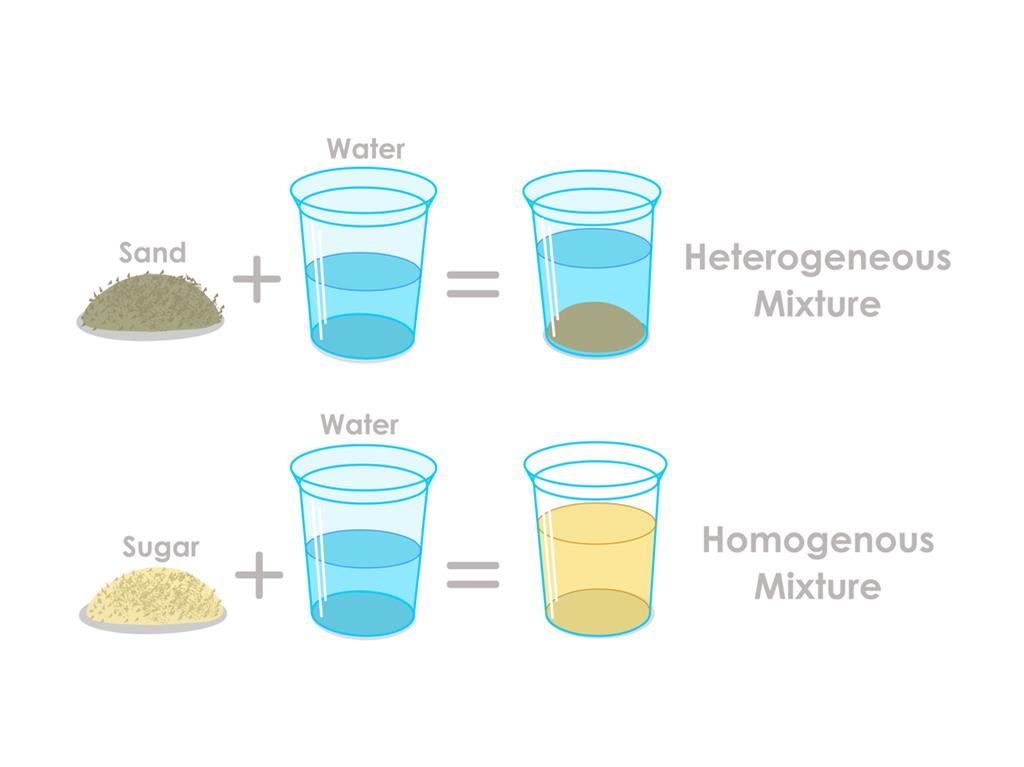
Are Table Salt Homogeneous Or Heterogeneous . By definition, a pure substance or a homogeneous mixture consists of a single phase. The answer to this query is straightforward: Explain the difference between a homogeneous mixture and a heterogeneous mixture. The difference is that the composition of the substance is always the same. Is table salt considered a homogeneous or heterogeneous substance? A heterogeneous mixture consists of two or more. Compounds are substances that are made up. Mixtures can be classified as homogeneous or heterogeneous. In other words, it possesses a uniform composition throughout,. Despite its homogeneity, table salt can vary in terms of its grain size or shape, iodine content, or the presence of additives. Table salt is a homogeneous substance because it has a. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. Elements and compounds are both examples of pure substances. The amount of salt in the salt water can vary from one sample to.
from www.yaclass.in
Is table salt considered a homogeneous or heterogeneous substance? A heterogeneous mixture consists of two or more. Table salt is a homogeneous substance because it has a. Explain the difference between a homogeneous mixture and a heterogeneous mixture. Mixtures can be classified as homogeneous or heterogeneous. Elements and compounds are both examples of pure substances. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. The amount of salt in the salt water can vary from one sample to. The answer to this query is straightforward: Compounds are substances that are made up.
Types of mixture Homogeneous and Heterogeneous — lesson. Science State
Are Table Salt Homogeneous Or Heterogeneous Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. A heterogeneous mixture consists of two or more. Despite its homogeneity, table salt can vary in terms of its grain size or shape, iodine content, or the presence of additives. Elements and compounds are both examples of pure substances. By definition, a pure substance or a homogeneous mixture consists of a single phase. The difference is that the composition of the substance is always the same. Explain the difference between a homogeneous mixture and a heterogeneous mixture. The answer to this query is straightforward: In other words, it possesses a uniform composition throughout,. Mixtures can be classified as homogeneous or heterogeneous. Is table salt considered a homogeneous or heterogeneous substance? Compounds are substances that are made up. The amount of salt in the salt water can vary from one sample to. Table salt is a homogeneous substance because it has a. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous.
From www.numerade.com
Activity 1 Check your understanding of mixtures Make mixtures of the Are Table Salt Homogeneous Or Heterogeneous The answer to this query is straightforward: The difference is that the composition of the substance is always the same. By definition, a pure substance or a homogeneous mixture consists of a single phase. Table salt is a homogeneous substance because it has a. Mixtures can be classified as homogeneous or heterogeneous. Compounds are substances that are made up. A. Are Table Salt Homogeneous Or Heterogeneous.
From www.thoughtco.com
10 Heterogeneous and Homogeneous Mixtures Are Table Salt Homogeneous Or Heterogeneous Despite its homogeneity, table salt can vary in terms of its grain size or shape, iodine content, or the presence of additives. The difference is that the composition of the substance is always the same. By definition, a pure substance or a homogeneous mixture consists of a single phase. Is table salt considered a homogeneous or heterogeneous substance? Explain the. Are Table Salt Homogeneous Or Heterogeneous.
From stock.adobe.com
Solution science experiment. Solubility of salt and sand in water Are Table Salt Homogeneous Or Heterogeneous Elements and compounds are both examples of pure substances. Explain the difference between a homogeneous mixture and a heterogeneous mixture. Table salt is a homogeneous substance because it has a. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. A heterogeneous mixture consists of two or more. By definition, a pure substance or a homogeneous. Are Table Salt Homogeneous Or Heterogeneous.
From jacksofscience.com
Is Salt Water a Heterogeneous Mixture? Jacks Of Science Are Table Salt Homogeneous Or Heterogeneous By definition, a pure substance or a homogeneous mixture consists of a single phase. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. Table salt is a homogeneous substance because it has a. Despite its homogeneity, table salt can vary in terms of its grain size or shape, iodine content, or the presence of additives.. Are Table Salt Homogeneous Or Heterogeneous.
From sciencenotes.org
10 Examples of Mixtures Are Table Salt Homogeneous Or Heterogeneous Explain the difference between a homogeneous mixture and a heterogeneous mixture. By definition, a pure substance or a homogeneous mixture consists of a single phase. The answer to this query is straightforward: The amount of salt in the salt water can vary from one sample to. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous.. Are Table Salt Homogeneous Or Heterogeneous.
From www.vectorstock.com
Mixture types experiment explanation Royalty Free Vector Are Table Salt Homogeneous Or Heterogeneous By definition, a pure substance or a homogeneous mixture consists of a single phase. The answer to this query is straightforward: Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. The amount of salt in the salt water can vary from one sample to. In other words, it possesses a uniform composition throughout,. The difference. Are Table Salt Homogeneous Or Heterogeneous.
From elchoroukhost.net
Is Table Salt A Heterogeneous Mixture Solution Compound Or Element Are Table Salt Homogeneous Or Heterogeneous In other words, it possesses a uniform composition throughout,. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. The answer to this query is straightforward: Compounds are substances that are made up. By definition, a pure substance or a homogeneous mixture consists of a single phase. Explain the difference between a homogeneous mixture and a. Are Table Salt Homogeneous Or Heterogeneous.
From slideplayer.com
Substances, Mixtures and Elements ppt download Are Table Salt Homogeneous Or Heterogeneous Elements and compounds are both examples of pure substances. By definition, a pure substance or a homogeneous mixture consists of a single phase. Table salt is a homogeneous substance because it has a. Compounds are substances that are made up. Is table salt considered a homogeneous or heterogeneous substance? Despite its homogeneity, table salt can vary in terms of its. Are Table Salt Homogeneous Or Heterogeneous.
From exyegvjoc.blob.core.windows.net
Table Salt Dissolved In Water A Homogeneous Or Heterogeneous Mixture at Are Table Salt Homogeneous Or Heterogeneous A heterogeneous mixture consists of two or more. Table salt is a homogeneous substance because it has a. Despite its homogeneity, table salt can vary in terms of its grain size or shape, iodine content, or the presence of additives. Explain the difference between a homogeneous mixture and a heterogeneous mixture. In other words, it possesses a uniform composition throughout,.. Are Table Salt Homogeneous Or Heterogeneous.
From msjschemclass.blogspot.com
Ms J's Chemistry Class Matter! Are Table Salt Homogeneous Or Heterogeneous Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. Is table salt considered a homogeneous or heterogeneous substance? Mixtures can be classified as homogeneous or heterogeneous. A heterogeneous mixture consists of two or more. Elements and compounds are both examples of pure substances. The amount of salt in the salt water can vary from one. Are Table Salt Homogeneous Or Heterogeneous.
From www.teachoo.com
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo Are Table Salt Homogeneous Or Heterogeneous Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. Compounds are substances that are made up. The answer to this query is straightforward: Is table salt considered a homogeneous or heterogeneous substance? Despite its homogeneity, table salt can vary in terms of its grain size or shape, iodine content, or the presence of additives. By. Are Table Salt Homogeneous Or Heterogeneous.
From www.slideshare.net
Elements, Compounds, Mixtures, Homogeneous, Are Table Salt Homogeneous Or Heterogeneous A heterogeneous mixture consists of two or more. Elements and compounds are both examples of pure substances. Despite its homogeneity, table salt can vary in terms of its grain size or shape, iodine content, or the presence of additives. The difference is that the composition of the substance is always the same. Compounds are substances that are made up. Table. Are Table Salt Homogeneous Or Heterogeneous.
From www.numerade.com
SOLVED Classify each of these as an element, a compound, a Are Table Salt Homogeneous Or Heterogeneous Compounds are substances that are made up. Mixtures can be classified as homogeneous or heterogeneous. Explain the difference between a homogeneous mixture and a heterogeneous mixture. The answer to this query is straightforward: A heterogeneous mixture consists of two or more. The difference is that the composition of the substance is always the same. The amount of salt in the. Are Table Salt Homogeneous Or Heterogeneous.
From fyojapcwx.blob.core.windows.net
Solution Of Table Salt In Glass Of Water Is Homogeneous at Isabell Are Table Salt Homogeneous Or Heterogeneous The difference is that the composition of the substance is always the same. Mixtures can be classified as homogeneous or heterogeneous. Elements and compounds are both examples of pure substances. In other words, it possesses a uniform composition throughout,. The answer to this query is straightforward: The amount of salt in the salt water can vary from one sample to.. Are Table Salt Homogeneous Or Heterogeneous.
From 88guru.com
What Are Homogeneous Mixtures Classification and Applications 88Guru Are Table Salt Homogeneous Or Heterogeneous Table salt is a homogeneous substance because it has a. The difference is that the composition of the substance is always the same. Compounds are substances that are made up. In other words, it possesses a uniform composition throughout,. By definition, a pure substance or a homogeneous mixture consists of a single phase. The amount of salt in the salt. Are Table Salt Homogeneous Or Heterogeneous.
From www.teachoo.com
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo Are Table Salt Homogeneous Or Heterogeneous The amount of salt in the salt water can vary from one sample to. Table salt is a homogeneous substance because it has a. In other words, it possesses a uniform composition throughout,. The difference is that the composition of the substance is always the same. By definition, a pure substance or a homogeneous mixture consists of a single phase.. Are Table Salt Homogeneous Or Heterogeneous.
From brainly.ph
salt and sugar homogeneous or heterogeneous Brainly.ph Are Table Salt Homogeneous Or Heterogeneous The amount of salt in the salt water can vary from one sample to. Is table salt considered a homogeneous or heterogeneous substance? Mixtures can be classified as homogeneous or heterogeneous. Despite its homogeneity, table salt can vary in terms of its grain size or shape, iodine content, or the presence of additives. The difference is that the composition of. Are Table Salt Homogeneous Or Heterogeneous.
From www.alamy.com
Solutions. homogeneous mixture. experiment with salt and water Are Table Salt Homogeneous Or Heterogeneous Mixtures can be classified as homogeneous or heterogeneous. A heterogeneous mixture consists of two or more. In other words, it possesses a uniform composition throughout,. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. Despite its homogeneity, table salt can vary in terms of its grain size or shape, iodine content, or the presence of. Are Table Salt Homogeneous Or Heterogeneous.
From nittygrittyscience.com
Section 1 Composition of Matter Nitty Gritty Science Are Table Salt Homogeneous Or Heterogeneous In other words, it possesses a uniform composition throughout,. Elements and compounds are both examples of pure substances. By definition, a pure substance or a homogeneous mixture consists of a single phase. A heterogeneous mixture consists of two or more. Compounds are substances that are made up. Classify a substance as an element, a compound, a homogeneous mixture, or a. Are Table Salt Homogeneous Or Heterogeneous.
From examples.yourdictionary.com
Examples of Heterogeneous Mixtures Types Made Simple YourDictionary Are Table Salt Homogeneous Or Heterogeneous Table salt is a homogeneous substance because it has a. Elements and compounds are both examples of pure substances. Is table salt considered a homogeneous or heterogeneous substance? Mixtures can be classified as homogeneous or heterogeneous. A heterogeneous mixture consists of two or more. The answer to this query is straightforward: Explain the difference between a homogeneous mixture and a. Are Table Salt Homogeneous Or Heterogeneous.
From pdfprof.com
is salty water homogeneous or heterogeneous Are Table Salt Homogeneous Or Heterogeneous Compounds are substances that are made up. Despite its homogeneity, table salt can vary in terms of its grain size or shape, iodine content, or the presence of additives. Is table salt considered a homogeneous or heterogeneous substance? The amount of salt in the salt water can vary from one sample to. A heterogeneous mixture consists of two or more.. Are Table Salt Homogeneous Or Heterogeneous.
From en.wikipedia.org
Homogeneous and heterogeneous mixtures Wikipedia Are Table Salt Homogeneous Or Heterogeneous Elements and compounds are both examples of pure substances. Explain the difference between a homogeneous mixture and a heterogeneous mixture. Mixtures can be classified as homogeneous or heterogeneous. The answer to this query is straightforward: Compounds are substances that are made up. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. By definition, a pure. Are Table Salt Homogeneous Or Heterogeneous.
From www.tutoroot.com
What are Heterogeneous and Homogeneous Mixtures? Tutoroot Are Table Salt Homogeneous Or Heterogeneous Despite its homogeneity, table salt can vary in terms of its grain size or shape, iodine content, or the presence of additives. Is table salt considered a homogeneous or heterogeneous substance? By definition, a pure substance or a homogeneous mixture consists of a single phase. Elements and compounds are both examples of pure substances. Classify a substance as an element,. Are Table Salt Homogeneous Or Heterogeneous.
From www.shutterstock.com
3,828 Salt Particles Images, Stock Photos & Vectors Shutterstock Are Table Salt Homogeneous Or Heterogeneous In other words, it possesses a uniform composition throughout,. The amount of salt in the salt water can vary from one sample to. Explain the difference between a homogeneous mixture and a heterogeneous mixture. Is table salt considered a homogeneous or heterogeneous substance? Mixtures can be classified as homogeneous or heterogeneous. By definition, a pure substance or a homogeneous mixture. Are Table Salt Homogeneous Or Heterogeneous.
From www.slideserve.com
PPT Why Study Chemistry? PowerPoint Presentation ID1267003 Are Table Salt Homogeneous Or Heterogeneous Mixtures can be classified as homogeneous or heterogeneous. The answer to this query is straightforward: Is table salt considered a homogeneous or heterogeneous substance? Compounds are substances that are made up. By definition, a pure substance or a homogeneous mixture consists of a single phase. Elements and compounds are both examples of pure substances. Classify a substance as an element,. Are Table Salt Homogeneous Or Heterogeneous.
From www.youtube.com
Homogeneous and Heterogeneous Mixtures Examples, Classification of Are Table Salt Homogeneous Or Heterogeneous Is table salt considered a homogeneous or heterogeneous substance? The difference is that the composition of the substance is always the same. Table salt is a homogeneous substance because it has a. Mixtures can be classified as homogeneous or heterogeneous. Explain the difference between a homogeneous mixture and a heterogeneous mixture. Classify a substance as an element, a compound, a. Are Table Salt Homogeneous Or Heterogeneous.
From www.youtube.com
Difference between Homogeneous mixture and pure Substance YouTube Are Table Salt Homogeneous Or Heterogeneous Mixtures can be classified as homogeneous or heterogeneous. A heterogeneous mixture consists of two or more. Despite its homogeneity, table salt can vary in terms of its grain size or shape, iodine content, or the presence of additives. Table salt is a homogeneous substance because it has a. Explain the difference between a homogeneous mixture and a heterogeneous mixture. The. Are Table Salt Homogeneous Or Heterogeneous.
From stock.adobe.com
3D Isometric Flat Vector Conceptual Illustration of Homogeneous And Are Table Salt Homogeneous Or Heterogeneous Explain the difference between a homogeneous mixture and a heterogeneous mixture. Compounds are substances that are made up. Is table salt considered a homogeneous or heterogeneous substance? Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. Table salt is a homogeneous substance because it has a. The answer to this query is straightforward: In other. Are Table Salt Homogeneous Or Heterogeneous.
From smartclass4kids.com
What is Mixture, Homogeneous Mixture, Heterogeneous Mixture with Examples Are Table Salt Homogeneous Or Heterogeneous Explain the difference between a homogeneous mixture and a heterogeneous mixture. The amount of salt in the salt water can vary from one sample to. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. Table salt is a homogeneous substance because it has a. The answer to this query is straightforward: In other words, it. Are Table Salt Homogeneous Or Heterogeneous.
From temperaturemaster.com
Is Table Salt a Compound, Mixture, or Solution? TempMaster Are Table Salt Homogeneous Or Heterogeneous Explain the difference between a homogeneous mixture and a heterogeneous mixture. The difference is that the composition of the substance is always the same. Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous. By definition, a pure substance or a homogeneous mixture consists of a single phase. The answer to this query is straightforward: Elements. Are Table Salt Homogeneous Or Heterogeneous.
From general.chemistrysteps.com
Pure Substances, Mixtures, Elements, and Compounds Chemistry Steps Are Table Salt Homogeneous Or Heterogeneous Elements and compounds are both examples of pure substances. Mixtures can be classified as homogeneous or heterogeneous. A heterogeneous mixture consists of two or more. Compounds are substances that are made up. The amount of salt in the salt water can vary from one sample to. Explain the difference between a homogeneous mixture and a heterogeneous mixture. Despite its homogeneity,. Are Table Salt Homogeneous Or Heterogeneous.
From www.chemicals.co.uk
What is a Mixture in Chemistry? The Chemistry Blog Are Table Salt Homogeneous Or Heterogeneous By definition, a pure substance or a homogeneous mixture consists of a single phase. Explain the difference between a homogeneous mixture and a heterogeneous mixture. The difference is that the composition of the substance is always the same. A heterogeneous mixture consists of two or more. Despite its homogeneity, table salt can vary in terms of its grain size or. Are Table Salt Homogeneous Or Heterogeneous.
From www.yaclass.in
Types of mixture Homogeneous and Heterogeneous — lesson. Science State Are Table Salt Homogeneous Or Heterogeneous The answer to this query is straightforward: A heterogeneous mixture consists of two or more. Explain the difference between a homogeneous mixture and a heterogeneous mixture. The amount of salt in the salt water can vary from one sample to. By definition, a pure substance or a homogeneous mixture consists of a single phase. In other words, it possesses a. Are Table Salt Homogeneous Or Heterogeneous.
From www.teachoo.com
Differentiate b/w Homogeneous and Heterogeneous mixtures Teachoo Are Table Salt Homogeneous Or Heterogeneous Despite its homogeneity, table salt can vary in terms of its grain size or shape, iodine content, or the presence of additives. By definition, a pure substance or a homogeneous mixture consists of a single phase. Table salt is a homogeneous substance because it has a. A heterogeneous mixture consists of two or more. Classify a substance as an element,. Are Table Salt Homogeneous Or Heterogeneous.
From techiescientist.com
Is Saltwater a Homogeneous or a Heterogeneous Mixture? Techiescientist Are Table Salt Homogeneous Or Heterogeneous The amount of salt in the salt water can vary from one sample to. Is table salt considered a homogeneous or heterogeneous substance? Despite its homogeneity, table salt can vary in terms of its grain size or shape, iodine content, or the presence of additives. Table salt is a homogeneous substance because it has a. Mixtures can be classified as. Are Table Salt Homogeneous Or Heterogeneous.