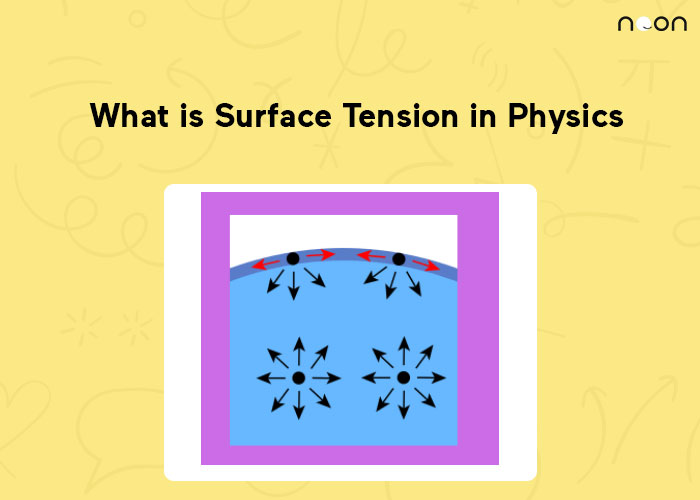
Why Surface Tension Is More Than Interfacial Tension . However the main forces involved in interfacial tension are adhesive forces. Note that surface tension increases. If the characteristic molecular dimension is r and its area thus r2, then the surface tension is σ ∼ u/(2r)2. Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. Surface tension is the property of the liquid in contact with gas phase (usually air). An example is the surface tension. While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. Interfacial tension, on the other hand, is the property between any two substances. Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. Interfacial tension is the tension force at the interface where two mediums meet. Surface tension is the tension at the boundary.
from www.learnatnoon.com
While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. If the characteristic molecular dimension is r and its area thus r2, then the surface tension is σ ∼ u/(2r)2. Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. Interfacial tension is the tension force at the interface where two mediums meet. Surface tension is the tension at the boundary. An example is the surface tension. Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. Note that surface tension increases. Interfacial tension, on the other hand, is the property between any two substances. Surface tension is the property of the liquid in contact with gas phase (usually air).
What is Surface Tension in Physics?
Why Surface Tension Is More Than Interfacial Tension Interfacial tension, on the other hand, is the property between any two substances. If the characteristic molecular dimension is r and its area thus r2, then the surface tension is σ ∼ u/(2r)2. Interfacial tension, on the other hand, is the property between any two substances. Surface tension is the property of the liquid in contact with gas phase (usually air). Surface tension is the tension at the boundary. Note that surface tension increases. Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. Interfacial tension is the tension force at the interface where two mediums meet. While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. However the main forces involved in interfacial tension are adhesive forces. Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. An example is the surface tension.
From www.nanoscience.com
Surface and Interfacial Tension How, What and Why? Nanoscience Why Surface Tension Is More Than Interfacial Tension An example is the surface tension. Note that surface tension increases. If the characteristic molecular dimension is r and its area thus r2, then the surface tension is σ ∼ u/(2r)2. Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. Interfacial tension, on the other hand, is the property between any two substances. Interfacial. Why Surface Tension Is More Than Interfacial Tension.
From www.worksheetsplanet.com
What is Surface Tension? Why Surface Tension Is More Than Interfacial Tension While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. Interfacial tension is the tension force at the interface where two mediums meet. If the characteristic molecular dimension is r and its area thus r2, then the surface tension is σ ∼ u/(2r)2. Typically,. Why Surface Tension Is More Than Interfacial Tension.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Why Surface Tension Is More Than Interfacial Tension An example is the surface tension. While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. Surface tension is the property of the liquid in contact with gas phase (usually air). Interfacial tension is the tension force at the interface where two mediums meet.. Why Surface Tension Is More Than Interfacial Tension.
From www.youtube.com
SURFACE TENSION & INTERFACIAL PHENOMENON PART1 INTERFACE TYPES Why Surface Tension Is More Than Interfacial Tension Surface tension is the tension at the boundary. Note that surface tension increases. Interfacial tension is the tension force at the interface where two mediums meet. Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. Surface tension is the property of the liquid in contact with gas phase (usually air). Interfacial tension, on the. Why Surface Tension Is More Than Interfacial Tension.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit Why Surface Tension Is More Than Interfacial Tension While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. Surface tension is the property of the liquid in contact with gas phase (usually air). An example is the surface tension. Interfacial tension is somewhat similar to surface tension in that cohesive forces are. Why Surface Tension Is More Than Interfacial Tension.
From hubpages.com
Surfactant Lowering Pulmonary Surface Tension Owlcation Why Surface Tension Is More Than Interfacial Tension While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. Surface tension is the property of the liquid in contact with gas phase (usually air). Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. However the main forces. Why Surface Tension Is More Than Interfacial Tension.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Why Surface Tension Is More Than Interfacial Tension Interfacial tension, on the other hand, is the property between any two substances. Surface tension is the property of the liquid in contact with gas phase (usually air). Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. Interfacial tension is the tension force at the interface where two mediums meet. Note. Why Surface Tension Is More Than Interfacial Tension.
From www.scribd.com
Surface Tension and Interfacial Phenomenon PDF Surface Tension Why Surface Tension Is More Than Interfacial Tension Note that surface tension increases. Surface tension is the tension at the boundary. However the main forces involved in interfacial tension are adhesive forces. If the characteristic molecular dimension is r and its area thus r2, then the surface tension is σ ∼ u/(2r)2. Interfacial tension, on the other hand, is the property between any two substances. An example is. Why Surface Tension Is More Than Interfacial Tension.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Why Surface Tension Is More Than Interfacial Tension Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. Interfacial tension, on the other hand, is the property between any two substances. Surface tension is the tension at. Why Surface Tension Is More Than Interfacial Tension.
From www.biolinscientific.com
Glossary Knowledge Biolin Scientific Why Surface Tension Is More Than Interfacial Tension Surface tension is the tension at the boundary. Interfacial tension, on the other hand, is the property between any two substances. An example is the surface tension. Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. While the term interfacial tension refers to interfaces between two liquids, the term surface tension. Why Surface Tension Is More Than Interfacial Tension.
From www.difference.wiki
Interfacial Tension vs. Surface Tension What’s the Difference? Why Surface Tension Is More Than Interfacial Tension If the characteristic molecular dimension is r and its area thus r2, then the surface tension is σ ∼ u/(2r)2. Interfacial tension, on the other hand, is the property between any two substances. Surface tension is the property of the liquid in contact with gas phase (usually air). Typically, the interfacial tension or interfacial (free) energy lies between the surface. Why Surface Tension Is More Than Interfacial Tension.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Why Surface Tension Is More Than Interfacial Tension An example is the surface tension. Note that surface tension increases. Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. Interfacial tension, on the other hand, is the property between any two substances. While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the. Why Surface Tension Is More Than Interfacial Tension.
From www.youtube.com
Surface & Interfacial Tension , part2 (surface tension & its unit Why Surface Tension Is More Than Interfacial Tension Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. An example is the surface tension. Interfacial tension is the tension force at the interface where two mediums meet. Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. Surface tension is the property of the liquid. Why Surface Tension Is More Than Interfacial Tension.
From schematicpartebriose.z14.web.core.windows.net
Concept Of Surface Tension Why Surface Tension Is More Than Interfacial Tension Note that surface tension increases. Interfacial tension is the tension force at the interface where two mediums meet. However the main forces involved in interfacial tension are adhesive forces. Interfacial tension, on the other hand, is the property between any two substances. Surface tension is the property of the liquid in contact with gas phase (usually air). Typically, the interfacial. Why Surface Tension Is More Than Interfacial Tension.
From www.quora.com
Why is surface tension greater than interfacial tension? Quora Why Surface Tension Is More Than Interfacial Tension However the main forces involved in interfacial tension are adhesive forces. Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. Surface tension is the property of the liquid in contact with gas phase (usually air). An example is the surface tension. If the characteristic molecular dimension is r and its area. Why Surface Tension Is More Than Interfacial Tension.
From www.slideshare.net
Surface and interfacial tension Why Surface Tension Is More Than Interfacial Tension Surface tension is the tension at the boundary. Note that surface tension increases. Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. Surface tension is the property of the liquid in contact with gas phase (usually air). While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to. Why Surface Tension Is More Than Interfacial Tension.
From blog.biolinscientific.com
What is surface tension? Why Surface Tension Is More Than Interfacial Tension However the main forces involved in interfacial tension are adhesive forces. Surface tension is the tension at the boundary. If the characteristic molecular dimension is r and its area thus r2, then the surface tension is σ ∼ u/(2r)2. Interfacial tension is the tension force at the interface where two mediums meet. Interfacial tension is somewhat similar to surface tension. Why Surface Tension Is More Than Interfacial Tension.
From scienceinfo.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences Why Surface Tension Is More Than Interfacial Tension Note that surface tension increases. While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. However the main forces involved in interfacial tension are adhesive forces. If the characteristic molecular dimension is r and its area thus r2, then the surface tension is σ. Why Surface Tension Is More Than Interfacial Tension.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Why Surface Tension Is More Than Interfacial Tension However the main forces involved in interfacial tension are adhesive forces. Interfacial tension, on the other hand, is the property between any two substances. Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the. Why Surface Tension Is More Than Interfacial Tension.
From www.slideshare.net
Surface and interfacial tension Why Surface Tension Is More Than Interfacial Tension Interfacial tension, on the other hand, is the property between any two substances. Surface tension is the tension at the boundary. Note that surface tension increases. Interfacial tension is the tension force at the interface where two mediums meet. Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. Surface tension is the property of. Why Surface Tension Is More Than Interfacial Tension.
From www.youtube.com
Difference between surface and interface surface tension and Why Surface Tension Is More Than Interfacial Tension An example is the surface tension. Surface tension is the tension at the boundary. Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. Note that surface tension increases. Interfacial tension is the tension force at the interface where two mediums meet. While the term interfacial tension refers to interfaces between two liquids, the term. Why Surface Tension Is More Than Interfacial Tension.
From courses.lumenlearning.com
Surface Tension Introduction to Chemistry Why Surface Tension Is More Than Interfacial Tension However the main forces involved in interfacial tension are adhesive forces. While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. An example is the surface tension. Interfacial tension,. Why Surface Tension Is More Than Interfacial Tension.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation Why Surface Tension Is More Than Interfacial Tension Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. Surface tension is the property of the liquid in contact with gas phase (usually air). Note that surface tension increases. While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous. Why Surface Tension Is More Than Interfacial Tension.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Why Surface Tension Is More Than Interfacial Tension Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. An example is the surface tension. Interfacial tension, on the other hand, is the property between any two substances. Note that surface tension increases. However the main forces involved in interfacial tension are adhesive forces. Interfacial tension is somewhat similar to surface. Why Surface Tension Is More Than Interfacial Tension.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Why Surface Tension Is More Than Interfacial Tension An example is the surface tension. Interfacial tension, on the other hand, is the property between any two substances. However the main forces involved in interfacial tension are adhesive forces. Surface tension is the property of the liquid in contact with gas phase (usually air). While the term interfacial tension refers to interfaces between two liquids, the term surface tension. Why Surface Tension Is More Than Interfacial Tension.
From www.learnatnoon.com
What is Surface Tension in Physics? Why Surface Tension Is More Than Interfacial Tension Surface tension is the property of the liquid in contact with gas phase (usually air). An example is the surface tension. Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. Surface tension is the tension at. Why Surface Tension Is More Than Interfacial Tension.
From byjus.com
Explain the surface tension phenomenon with examples. Why Surface Tension Is More Than Interfacial Tension Surface tension is the property of the liquid in contact with gas phase (usually air). An example is the surface tension. Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. Interfacial tension is the tension force at the interface where two mediums meet. Surface tension is the tension at the boundary.. Why Surface Tension Is More Than Interfacial Tension.
From www.askdifference.com
Interfacial Tension vs. Surface Tension — What’s the Difference? Why Surface Tension Is More Than Interfacial Tension While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. Note that surface tension increases. An example is the surface tension. If the characteristic molecular dimension is r and its area thus r2, then the surface tension is σ ∼ u/(2r)2. Surface tension is. Why Surface Tension Is More Than Interfacial Tension.
From www.slideserve.com
PPT Interfacial Phenomena PowerPoint Presentation, free download ID Why Surface Tension Is More Than Interfacial Tension Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. Surface tension is the tension at the boundary. If the characteristic molecular dimension is r and its area thus r2, then the surface tension is σ ∼ u/(2r)2. Interfacial tension is the tension force at the interface where two mediums meet. However the main forces. Why Surface Tension Is More Than Interfacial Tension.
From guidemanualspyglasses.z14.web.core.windows.net
How To Explain Surface Tension Why Surface Tension Is More Than Interfacial Tension Note that surface tension increases. Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. Surface tension is the tension at the boundary. Interfacial tension is. Why Surface Tension Is More Than Interfacial Tension.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru Why Surface Tension Is More Than Interfacial Tension Surface tension is the tension at the boundary. Interfacial tension is the tension force at the interface where two mediums meet. Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a. Why Surface Tension Is More Than Interfacial Tension.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Why Surface Tension Is More Than Interfacial Tension Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. Surface tension is the property of the liquid in contact with gas phase (usually air). Interfacial tension, on the other hand, is the property between any two substances. An example is the surface tension. Interfacial tension is somewhat similar to surface tension. Why Surface Tension Is More Than Interfacial Tension.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID6036532 Why Surface Tension Is More Than Interfacial Tension Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. If the characteristic molecular dimension is r and its area thus r2, then the surface tension is σ ∼ u/(2r)2. Surface tension is the property of the liquid in contact with gas phase (usually air). Surface tension is the tension at the boundary. An example. Why Surface Tension Is More Than Interfacial Tension.
From askanydifference.com
Tensión interfacial frente a tensión superficial diferencia y comparación Why Surface Tension Is More Than Interfacial Tension However the main forces involved in interfacial tension are adhesive forces. Interfacial tension is the tension force at the interface where two mediums meet. If the characteristic molecular dimension is r and its area thus r2, then the surface tension is σ ∼ u/(2r)2. Interfacial tension, on the other hand, is the property between any two substances. Surface tension is. Why Surface Tension Is More Than Interfacial Tension.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL Why Surface Tension Is More Than Interfacial Tension Typically, the interfacial tension or interfacial (free) energy lies between the surface tensions of the individual condensed phases. Surface tension is the property of the liquid in contact with gas phase (usually air). Interfacial tension is the tension force at the interface where two mediums meet. An example is the surface tension. Surface tension is the tension at the boundary.. Why Surface Tension Is More Than Interfacial Tension.