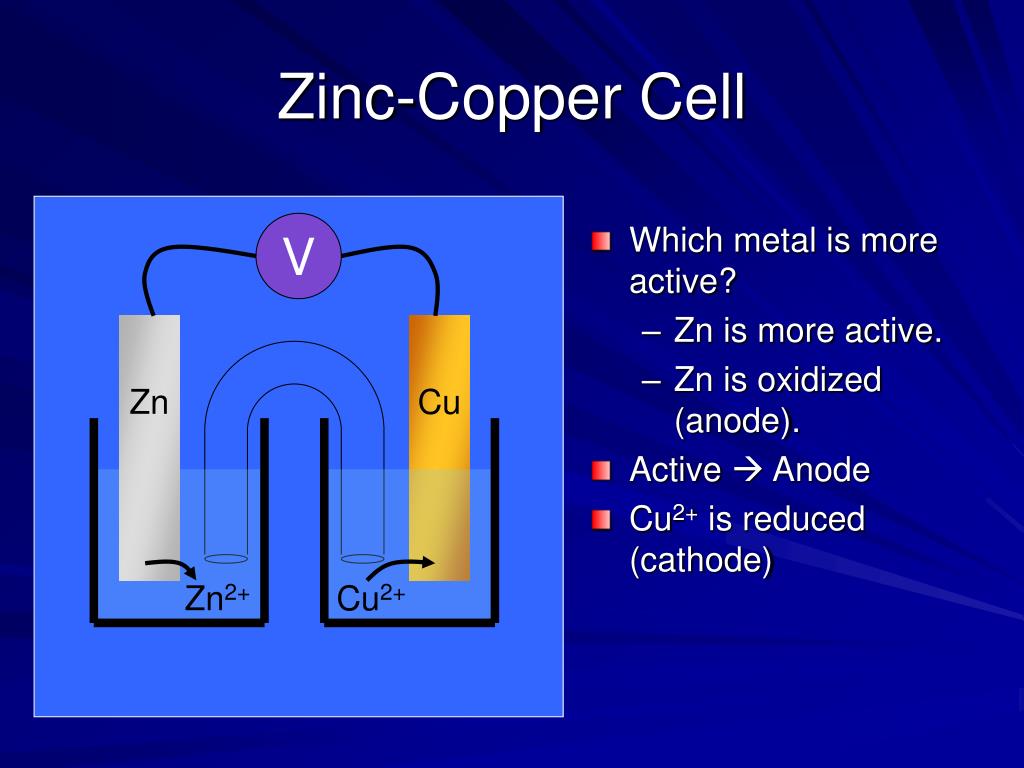
Zinc And Copper Cell Notation . use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. Sometimes the state of each species into the cell is written. the moment current flows [zn +2] increases while the [cu +2] decreases and this can be seen by the images in part (b) of figure. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. zinc is oxidized, and copper ions are reduced. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved. zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons.
from www.slideserve.com
use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. Sometimes the state of each species into the cell is written. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. zinc is oxidized, and copper ions are reduced. the moment current flows [zn +2] increases while the [cu +2] decreases and this can be seen by the images in part (b) of figure. zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the.
PPT Electrochemical Cells PowerPoint Presentation, free download ID
Zinc And Copper Cell Notation zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved. Sometimes the state of each species into the cell is written. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. the moment current flows [zn +2] increases while the [cu +2] decreases and this can be seen by the images in part (b) of figure. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. zinc is oxidized, and copper ions are reduced.
From slideplayer.com
Electrochemistry Redox Reactions and Electrochemical Cells ppt download Zinc And Copper Cell Notation Sometimes the state of each species into the cell is written. the moment current flows [zn +2] increases while the [cu +2] decreases and this can be seen by the images in part (b) of figure. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. a typical cell. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Zinc And Copper Cell Notation Sometimes the state of each species into the cell is written. zinc is oxidized, and copper ions are reduced. zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. use cell notation to describe the galvanic cell where copper(ii) ions are reduced. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT Electrochemistry Part II The Galvanic Cell PowerPoint Zinc And Copper Cell Notation a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. the moment current flows [zn +2] increases while the [cu +2] decreases and this. Zinc And Copper Cell Notation.
From www.numerade.com
SOLVED Use cell notation to describe the galvanic cell where copper(II Zinc And Copper Cell Notation zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. the moment current flows [zn +2] increases while the [cu +2] decreases and this can be seen by the images in part (b) of figure. use cell notation to describe the galvanic. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2810901 Zinc And Copper Cell Notation a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved. zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. zinc is oxidized, and copper ions are. Zinc And Copper Cell Notation.
From www.youtube.com
Electrochemical cell Zn and Cu YouTube Zinc And Copper Cell Notation use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. a typical cell might consist of two pieces of metal,. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2810901 Zinc And Copper Cell Notation zinc is oxidized, and copper ions are reduced. Sometimes the state of each species into the cell is written. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Zinc And Copper Cell Notation a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. use cell notation to. Zinc And Copper Cell Notation.
From slideplayer.com
Chem 132 General Chemistry II ppt download Zinc And Copper Cell Notation a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. zinc is oxidized, and copper ions are. Zinc And Copper Cell Notation.
From stock.adobe.com
Illustration of galvanic cell consists of zinc and copper. Stock Vector Zinc And Copper Cell Notation zinc is oxidized, and copper ions are reduced. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. the moment current flows [zn +2] increases while. Zinc And Copper Cell Notation.
From blog.thepipingmart.com
Classifications of Metals Copper vs. Zinc Zinc And Copper Cell Notation Sometimes the state of each species into the cell is written. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. . Zinc And Copper Cell Notation.
From www.numerade.com
SOLVED Text Modified Copper and Zinc Voltaic Cell Write the cell Zinc And Copper Cell Notation Sometimes the state of each species into the cell is written. zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. zinc is oxidized, and copper ions are reduced. a typical cell might consist of two pieces of metal, one zinc and. Zinc And Copper Cell Notation.
From slideplayer.com
Electrochemistry. ppt download Zinc And Copper Cell Notation zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. zinc is oxidized, and copper ions are reduced. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. a typical cell might consist. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5405206 Zinc And Copper Cell Notation use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. a typical cell might consist of two pieces of metal, one zinc and the other copper, each. Zinc And Copper Cell Notation.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Zinc And Copper Cell Notation use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved. zinc is oxidized, and copper ions are reduced. use cell notation to describe. Zinc And Copper Cell Notation.
From www.chegg.com
Solved C. Copper and Zinc Voltaic Cell 1. Write the cell Zinc And Copper Cell Notation zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. the moment current flows [zn +2] increases while the [cu +2] decreases and this can be seen by the images in part (b) of figure. zinc is oxidized, and copper ions are. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT Chapter Twenty PowerPoint Presentation, free download ID5368420 Zinc And Copper Cell Notation a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved. zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. Sometimes the state of each species into the. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Zinc And Copper Cell Notation a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. Sometimes the state of each species into the cell is written. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each. Zinc And Copper Cell Notation.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Copper Zinc And Copper Cell Notation use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. the moment current flows [zn +2] increases while the [cu +2] decreases and this can be seen. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT What is an Electrochemical Cell? PowerPoint Presentation, free Zinc And Copper Cell Notation zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved. use cell notation to describe the galvanic. Zinc And Copper Cell Notation.
From slideplayer.com
Electrochemistry Chapter ppt download Zinc And Copper Cell Notation use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved. zinc is oxidized, and copper ions are reduced. a. Zinc And Copper Cell Notation.
From blog.thepipingmart.com
Difference Between Copper and Zinc Zinc And Copper Cell Notation the moment current flows [zn +2] increases while the [cu +2] decreases and this can be seen by the images in part (b) of figure. zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. use cell notation to describe the galvanic. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT Cells and Voltage PowerPoint Presentation ID5231819 Zinc And Copper Cell Notation a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved. zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. use cell notation to describe the galvanic. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Zinc And Copper Cell Notation a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved. the moment current flows [zn +2] increases while the [cu +2] decreases and this can be seen by the images in part (b) of figure. use cell notation to describe the galvanic. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Zinc And Copper Cell Notation a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. zinc is oxidized, and copper ions are reduced. zinc atoms. Zinc And Copper Cell Notation.
From ar.inspiredpencil.com
Copper Electrolytic Cell Zinc And Copper Cell Notation use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. Sometimes the state of each species into the cell is written. a typical cell might consist of. Zinc And Copper Cell Notation.
From www.youtube.com
Determination of Standard Electrode potential of Zinc and Copper YouTube Zinc And Copper Cell Notation the moment current flows [zn +2] increases while the [cu +2] decreases and this can be seen by the images in part (b) of figure. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. zinc atoms on the. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT Chapter Twenty PowerPoint Presentation, free download ID5368420 Zinc And Copper Cell Notation use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. zinc is oxidized, and copper ions are reduced. Sometimes the state of each species into the cell. Zinc And Copper Cell Notation.
From slideplayer.com
Energy in a cell Today, we will learn to figure out how much energy we Zinc And Copper Cell Notation a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. zinc is oxidized, and copper ions are reduced. zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind. Zinc And Copper Cell Notation.
From wisc.pb.unizin.org
D40.2 Cell Notation Chemistry 109 Fall 2021 Zinc And Copper Cell Notation zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. a typical cell might consist of two pieces of metal, one zinc and the. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation ID1195562 Zinc And Copper Cell Notation zinc is oxidized, and copper ions are reduced. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. the. Zinc And Copper Cell Notation.
From www.numerade.com
SOLVEDCell Iype Anode Cathode_Metall Cell _Shorthand Notation Metall Zinc And Copper Cell Notation use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. Sometimes the state of each species into the cell is written. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved. Zinc And Copper Cell Notation.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Zinc And Copper Cell Notation a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. zinc is oxidized, and copper ions are reduced. a typical. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1195570 Zinc And Copper Cell Notation Sometimes the state of each species into the cell is written. the moment current flows [zn +2] increases while the [cu +2] decreases and this can be seen by the images in part (b) of figure. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution. Zinc And Copper Cell Notation.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID6754031 Zinc And Copper Cell Notation zinc atoms on the solid piece of zinc are released into the solution as z n 2 + ions, leaving behind their two valence electrons. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. a typical cell might. Zinc And Copper Cell Notation.