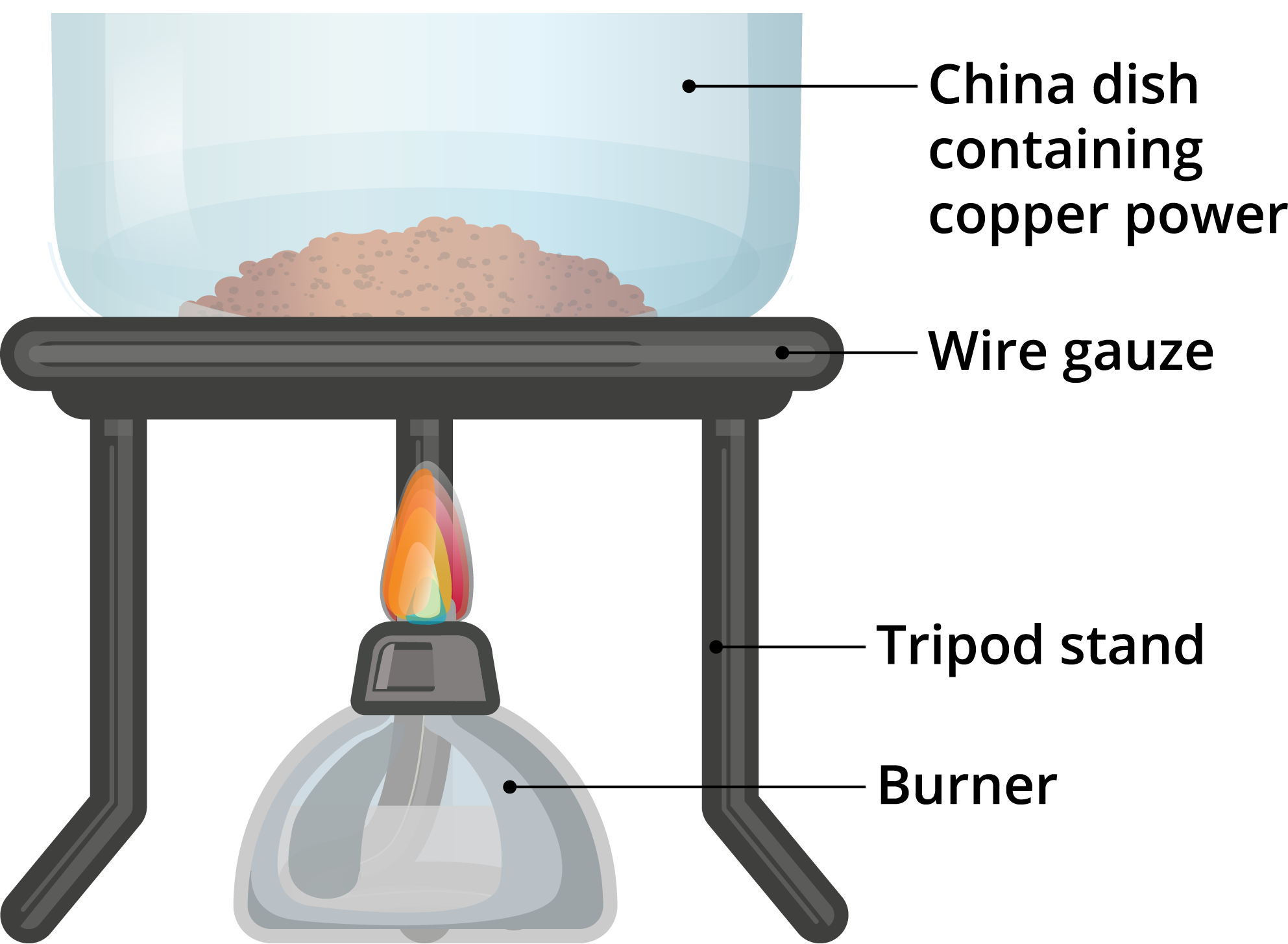
Iron Heated With Copper(Ii) Oxide . We say that it has been reduced to copper. The copper oxide can then react with the hydrogen gas to form the copper. When solid copper (i) oxide is heated with hydrogen, for example, its mass decreases because the formation of pure copper is accompanied by the loss of oxygen atoms as a. At each stage, students look. Carbon is above iron in the reactivity series, and will take oxygen from iron. It isn't actually used industrially to extract copper from copper ores, but is used to extract iron from iron oxide ores. In some cases, the oxide formed is more complicated than you. In the equation, the copper (ii) oxide has lost its oxygen. Heated copper metal reacts with oxygen to form the black copper oxide. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. The compound, copper oxide, is formed on the surface of the metal. Copper oxide is black and in time will coat the surface of copper left in air. Oxidation and reduction always go hand. Copper doesn't burn, but its surface is converted to black copper (ii) oxide.
from www.yaclass.in
Oxidation and reduction always go hand. It isn't actually used industrially to extract copper from copper ores, but is used to extract iron from iron oxide ores. Copper oxide is black and in time will coat the surface of copper left in air. At each stage, students look. The copper oxide can then react with the hydrogen gas to form the copper. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. When solid copper (i) oxide is heated with hydrogen, for example, its mass decreases because the formation of pure copper is accompanied by the loss of oxygen atoms as a. In some cases, the oxide formed is more complicated than you. Carbon is above iron in the reactivity series, and will take oxygen from iron. In the equation, the copper (ii) oxide has lost its oxygen.
Oxidation and Reduction — lesson. Science CBSE, Class 10.
Iron Heated With Copper(Ii) Oxide Heated copper metal reacts with oxygen to form the black copper oxide. In some cases, the oxide formed is more complicated than you. Copper oxide is black and in time will coat the surface of copper left in air. When solid copper (i) oxide is heated with hydrogen, for example, its mass decreases because the formation of pure copper is accompanied by the loss of oxygen atoms as a. It isn't actually used industrially to extract copper from copper ores, but is used to extract iron from iron oxide ores. Copper doesn't burn, but its surface is converted to black copper (ii) oxide. We say that it has been reduced to copper. In the equation, the copper (ii) oxide has lost its oxygen. Heated copper metal reacts with oxygen to form the black copper oxide. At each stage, students look. Carbon is above iron in the reactivity series, and will take oxygen from iron. Oxidation and reduction always go hand. The copper oxide can then react with the hydrogen gas to form the copper. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. The compound, copper oxide, is formed on the surface of the metal.
From www.chegg.com
Solved Correct PartC olid copper (II) oxide reacts with Iron Heated With Copper(Ii) Oxide The compound, copper oxide, is formed on the surface of the metal. It isn't actually used industrially to extract copper from copper ores, but is used to extract iron from iron oxide ores. Oxidation and reduction always go hand. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position. Iron Heated With Copper(Ii) Oxide.
From edu.rsc.org
Reacting copper(II) oxide with sulfuric acid Experiment RSC Education Iron Heated With Copper(Ii) Oxide Copper oxide is black and in time will coat the surface of copper left in air. The copper oxide can then react with the hydrogen gas to form the copper. Oxidation and reduction always go hand. In the equation, the copper (ii) oxide has lost its oxygen. Copper doesn't burn, but its surface is converted to black copper (ii) oxide.. Iron Heated With Copper(Ii) Oxide.
From www.youtube.com
Heating a mixture of copper (II) oxide and carbon C0118 YouTube Iron Heated With Copper(Ii) Oxide The copper oxide can then react with the hydrogen gas to form the copper. Copper oxide is black and in time will coat the surface of copper left in air. Oxidation and reduction always go hand. When solid copper (i) oxide is heated with hydrogen, for example, its mass decreases because the formation of pure copper is accompanied by the. Iron Heated With Copper(Ii) Oxide.
From questions-in.kunduz.com
What happens when copper is heated in air... Physical Chemistry Iron Heated With Copper(Ii) Oxide Carbon is above iron in the reactivity series, and will take oxygen from iron. Copper doesn't burn, but its surface is converted to black copper (ii) oxide. The copper oxide can then react with the hydrogen gas to form the copper. In some cases, the oxide formed is more complicated than you. We say that it has been reduced to. Iron Heated With Copper(Ii) Oxide.
From www.coursehero.com
[Solved] 1. When solid copper (II) oxide is heated in the presence of Iron Heated With Copper(Ii) Oxide When solid copper (i) oxide is heated with hydrogen, for example, its mass decreases because the formation of pure copper is accompanied by the loss of oxygen atoms as a. Oxidation and reduction always go hand. Carbon is above iron in the reactivity series, and will take oxygen from iron. In the equation, the copper (ii) oxide has lost its. Iron Heated With Copper(Ii) Oxide.
From www.slideserve.com
PPT Writing Formulas and Names of Ionic Compounds PowerPoint Iron Heated With Copper(Ii) Oxide In some cases, the oxide formed is more complicated than you. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. Heated copper metal reacts with oxygen to form the black copper oxide. Carbon is above iron in the reactivity series, and will take oxygen. Iron Heated With Copper(Ii) Oxide.
From www.doubtnut.com
When hydrogen gas is passed over heated copper (II) oxide, copper and Iron Heated With Copper(Ii) Oxide The compound, copper oxide, is formed on the surface of the metal. Copper doesn't burn, but its surface is converted to black copper (ii) oxide. It isn't actually used industrially to extract copper from copper ores, but is used to extract iron from iron oxide ores. Copper oxide is black and in time will coat the surface of copper left. Iron Heated With Copper(Ii) Oxide.
From fphoto.photoshelter.com
science chemistry oxidation reaction cupric oxide Fundamental Iron Heated With Copper(Ii) Oxide Copper oxide is black and in time will coat the surface of copper left in air. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. In some cases, the oxide formed is more complicated than you. It isn't actually used industrially to extract copper. Iron Heated With Copper(Ii) Oxide.
From fphoto.com
Fundamental Photographs The Art of Science Iron Heated With Copper(Ii) Oxide In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. We say that it has been reduced to copper. It isn't actually used industrially to extract copper from copper ores, but is used to extract iron from iron oxide ores. Heated copper metal reacts with. Iron Heated With Copper(Ii) Oxide.
From www.alamy.com
Copper (II) oxide (CuO). This chemical contains copper in a +2 Iron Heated With Copper(Ii) Oxide In some cases, the oxide formed is more complicated than you. Copper oxide is black and in time will coat the surface of copper left in air. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. Carbon is above iron in the reactivity series,. Iron Heated With Copper(Ii) Oxide.
From atelier-yuwa.ciao.jp
Finding The Formula Of Copper(II) Oxide Experiment RSC Education Iron Heated With Copper(Ii) Oxide The copper oxide can then react with the hydrogen gas to form the copper. Oxidation and reduction always go hand. In the equation, the copper (ii) oxide has lost its oxygen. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. Copper oxide is black. Iron Heated With Copper(Ii) Oxide.
From www.youtube.com
On heating blue coloured powder of copper (II) nitrate in a boiling Iron Heated With Copper(Ii) Oxide Oxidation and reduction always go hand. At each stage, students look. Heated copper metal reacts with oxygen to form the black copper oxide. When solid copper (i) oxide is heated with hydrogen, for example, its mass decreases because the formation of pure copper is accompanied by the loss of oxygen atoms as a. Copper doesn't burn, but its surface is. Iron Heated With Copper(Ii) Oxide.
From www.sciencephoto.com
Copper (II) oxide Stock Image C014/6955 Science Photo Library Iron Heated With Copper(Ii) Oxide Copper oxide is black and in time will coat the surface of copper left in air. It isn't actually used industrially to extract copper from copper ores, but is used to extract iron from iron oxide ores. The compound, copper oxide, is formed on the surface of the metal. The copper oxide can then react with the hydrogen gas to. Iron Heated With Copper(Ii) Oxide.
From www.hanlin.com
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:1.5.7 Practical Determine Iron Heated With Copper(Ii) Oxide We say that it has been reduced to copper. It isn't actually used industrially to extract copper from copper ores, but is used to extract iron from iron oxide ores. Heated copper metal reacts with oxygen to form the black copper oxide. When solid copper (i) oxide is heated with hydrogen, for example, its mass decreases because the formation of. Iron Heated With Copper(Ii) Oxide.
From www.sciencephoto.com
Copper (II) oxide Stock Image C007/0865 Science Photo Library Iron Heated With Copper(Ii) Oxide In some cases, the oxide formed is more complicated than you. The copper oxide can then react with the hydrogen gas to form the copper. It isn't actually used industrially to extract copper from copper ores, but is used to extract iron from iron oxide ores. Copper doesn't burn, but its surface is converted to black copper (ii) oxide. Oxidation. Iron Heated With Copper(Ii) Oxide.
From www.sciencephoto.com
Copper (II) oxide reacting with sulphuric acid Stock Image C052 Iron Heated With Copper(Ii) Oxide Oxidation and reduction always go hand. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. We say that it has been reduced to copper. When solid copper (i) oxide is heated with hydrogen, for example, its mass decreases because the formation of pure copper. Iron Heated With Copper(Ii) Oxide.
From www.youtube.com
Copper(ii) Oxide Reduction using Hydrogen YouTube Iron Heated With Copper(Ii) Oxide The copper oxide can then react with the hydrogen gas to form the copper. Carbon is above iron in the reactivity series, and will take oxygen from iron. Copper oxide is black and in time will coat the surface of copper left in air. In this experiment, students heat iron metal with the oxides of two other metals, copper and. Iron Heated With Copper(Ii) Oxide.
From www.slideshare.net
Action of heat on chemical compound e book Iron Heated With Copper(Ii) Oxide In some cases, the oxide formed is more complicated than you. Heated copper metal reacts with oxygen to form the black copper oxide. The copper oxide can then react with the hydrogen gas to form the copper. It isn't actually used industrially to extract copper from copper ores, but is used to extract iron from iron oxide ores. In the. Iron Heated With Copper(Ii) Oxide.
From www.slideserve.com
PPT CHAPTER 6 PHYSICAL AND CHEMICAL CHANGES PowerPoint Presentation Iron Heated With Copper(Ii) Oxide When solid copper (i) oxide is heated with hydrogen, for example, its mass decreases because the formation of pure copper is accompanied by the loss of oxygen atoms as a. The compound, copper oxide, is formed on the surface of the metal. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to. Iron Heated With Copper(Ii) Oxide.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:2.3.4 Carbon Dioxide from Iron Heated With Copper(Ii) Oxide The compound, copper oxide, is formed on the surface of the metal. The copper oxide can then react with the hydrogen gas to form the copper. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. It isn't actually used industrially to extract copper from. Iron Heated With Copper(Ii) Oxide.
From www.youtube.com
Chemical Properties of Hydrogen Reaction of hydrogen with copper II Iron Heated With Copper(Ii) Oxide In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. At each stage, students look. The copper oxide can then react with the hydrogen gas to form the copper. In some cases, the oxide formed is more complicated than you. The compound, copper oxide, is. Iron Heated With Copper(Ii) Oxide.
From edu.rsc.org
Reduction of copper(II) oxide by hydrogen Experiment RSC Education Iron Heated With Copper(Ii) Oxide Heated copper metal reacts with oxygen to form the black copper oxide. It isn't actually used industrially to extract copper from copper ores, but is used to extract iron from iron oxide ores. Copper oxide is black and in time will coat the surface of copper left in air. When solid copper (i) oxide is heated with hydrogen, for example,. Iron Heated With Copper(Ii) Oxide.
From edu-rsc-org-s.webvpn.bjmu.doc110.com
Finding the formula of copper(II) oxide Experiment RSC Education Iron Heated With Copper(Ii) Oxide We say that it has been reduced to copper. At each stage, students look. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. It isn't actually used industrially to extract copper from copper ores, but is used to extract iron from iron oxide ores.. Iron Heated With Copper(Ii) Oxide.
From www.online-sciences.com
Types of chemical reactions and Thermal reactions Iron Heated With Copper(Ii) Oxide Heated copper metal reacts with oxygen to form the black copper oxide. Oxidation and reduction always go hand. The copper oxide can then react with the hydrogen gas to form the copper. When solid copper (i) oxide is heated with hydrogen, for example, its mass decreases because the formation of pure copper is accompanied by the loss of oxygen atoms. Iron Heated With Copper(Ii) Oxide.
From www.numerade.com
SOLVED When copper(II) oxide is heated in hydrogen gas, the following Iron Heated With Copper(Ii) Oxide In some cases, the oxide formed is more complicated than you. Copper doesn't burn, but its surface is converted to black copper (ii) oxide. The copper oxide can then react with the hydrogen gas to form the copper. It isn't actually used industrially to extract copper from copper ores, but is used to extract iron from iron oxide ores. Copper. Iron Heated With Copper(Ii) Oxide.
From www.sciencephoto.com
Copper (II) Oxide Stock Image C028/0885 Science Photo Library Iron Heated With Copper(Ii) Oxide We say that it has been reduced to copper. The copper oxide can then react with the hydrogen gas to form the copper. The compound, copper oxide, is formed on the surface of the metal. Copper doesn't burn, but its surface is converted to black copper (ii) oxide. Heated copper metal reacts with oxygen to form the black copper oxide.. Iron Heated With Copper(Ii) Oxide.
From www.yaclass.in
Oxidation and Reduction — lesson. Science CBSE, Class 10. Iron Heated With Copper(Ii) Oxide Oxidation and reduction always go hand. Copper doesn't burn, but its surface is converted to black copper (ii) oxide. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. When solid copper (i) oxide is heated with hydrogen, for example, its mass decreases because the. Iron Heated With Copper(Ii) Oxide.
From www.researchgate.net
(PDF) Capturing of the copper(II) ions by several 4aminoantipyrine Iron Heated With Copper(Ii) Oxide At each stage, students look. Heated copper metal reacts with oxygen to form the black copper oxide. The copper oxide can then react with the hydrogen gas to form the copper. We say that it has been reduced to copper. Copper doesn't burn, but its surface is converted to black copper (ii) oxide. In the equation, the copper (ii) oxide. Iron Heated With Copper(Ii) Oxide.
From www.science-revision.co.uk
Extracting metals Iron Heated With Copper(Ii) Oxide Heated copper metal reacts with oxygen to form the black copper oxide. At each stage, students look. Copper doesn't burn, but its surface is converted to black copper (ii) oxide. Oxidation and reduction always go hand. The compound, copper oxide, is formed on the surface of the metal. It isn't actually used industrially to extract copper from copper ores, but. Iron Heated With Copper(Ii) Oxide.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Iron Heated With Copper(Ii) Oxide Copper doesn't burn, but its surface is converted to black copper (ii) oxide. At each stage, students look. Oxidation and reduction always go hand. Heated copper metal reacts with oxygen to form the black copper oxide. Carbon is above iron in the reactivity series, and will take oxygen from iron. When solid copper (i) oxide is heated with hydrogen, for. Iron Heated With Copper(Ii) Oxide.
From dokumen.tips
(PDF) The thermal of copper (II) oxalate revisited · The Iron Heated With Copper(Ii) Oxide The copper oxide can then react with the hydrogen gas to form the copper. When solid copper (i) oxide is heated with hydrogen, for example, its mass decreases because the formation of pure copper is accompanied by the loss of oxygen atoms as a. Copper doesn't burn, but its surface is converted to black copper (ii) oxide. In this experiment,. Iron Heated With Copper(Ii) Oxide.
From www.toppr.com
9 Copper(t) carbonate is broken down by heating to form copper(11 Iron Heated With Copper(Ii) Oxide The copper oxide can then react with the hydrogen gas to form the copper. Copper doesn't burn, but its surface is converted to black copper (ii) oxide. At each stage, students look. The compound, copper oxide, is formed on the surface of the metal. It isn't actually used industrially to extract copper from copper ores, but is used to extract. Iron Heated With Copper(Ii) Oxide.
From www.youtube.com
Heating a mixture of carbon and copper (II) oxide YouTube Iron Heated With Copper(Ii) Oxide It isn't actually used industrially to extract copper from copper ores, but is used to extract iron from iron oxide ores. Oxidation and reduction always go hand. At each stage, students look. In this experiment, students heat iron metal with the oxides of two other metals, copper and magnesium, to determine its position in the reactivity series. Copper oxide is. Iron Heated With Copper(Ii) Oxide.
From edu.rsc.org
Reacting copper(II) oxide with sulfuric acid Experiment RSC Education Iron Heated With Copper(Ii) Oxide Carbon is above iron in the reactivity series, and will take oxygen from iron. When solid copper (i) oxide is heated with hydrogen, for example, its mass decreases because the formation of pure copper is accompanied by the loss of oxygen atoms as a. Heated copper metal reacts with oxygen to form the black copper oxide. In some cases, the. Iron Heated With Copper(Ii) Oxide.
From www.flickr.com
Copper formed after reduction of copper (II) oxide when heated with Iron Heated With Copper(Ii) Oxide It isn't actually used industrially to extract copper from copper ores, but is used to extract iron from iron oxide ores. The compound, copper oxide, is formed on the surface of the metal. Copper oxide is black and in time will coat the surface of copper left in air. The copper oxide can then react with the hydrogen gas to. Iron Heated With Copper(Ii) Oxide.