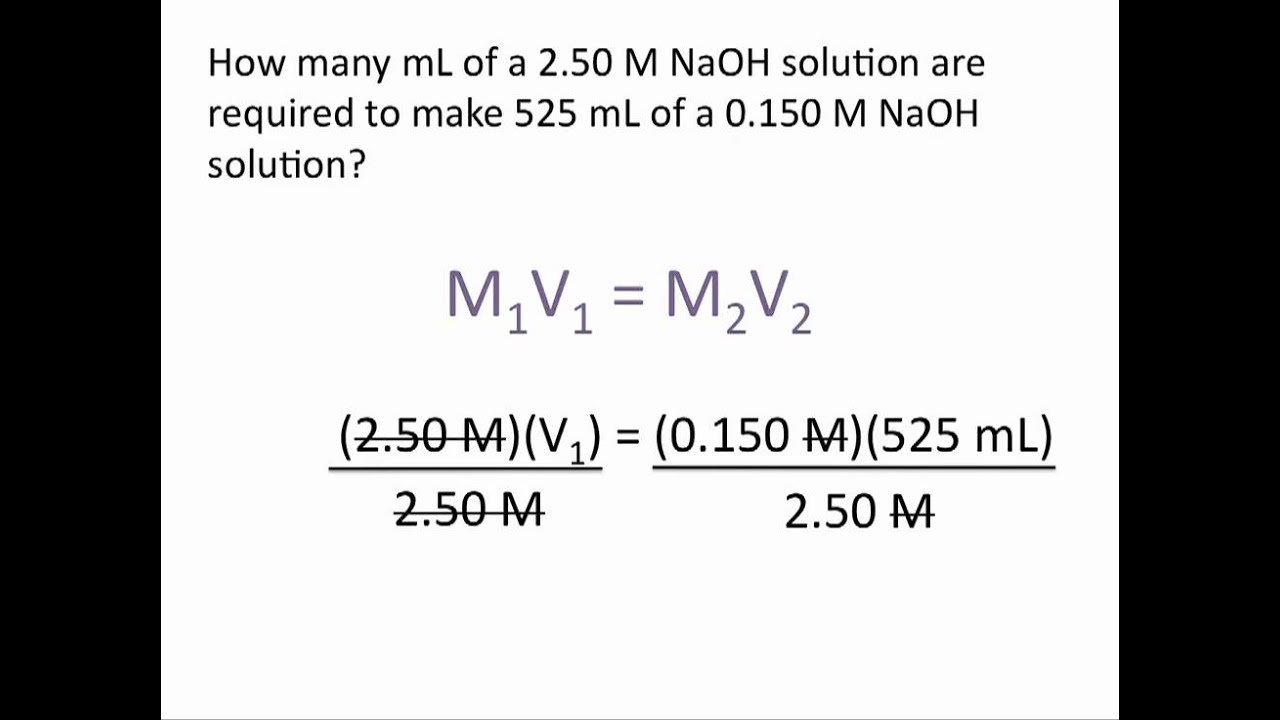
Dilution And Concentration Problems . Dilution is the addition of. What is the new concentration? How much of it do you need to prepare 50 ml of a. The concentration and the volumes change in a dilution. When dealing with dilutions such as those in questions 5 and 6, a handy trick can be used. There’s a bottle of 0.750 m nacl on a shelf. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. If the concentration and volume of the initial solution are c1 and v1. State whether the concentration of a solution is directly or indirectly proportional to its volume. This chemistry video tutorial explains how to solve common dilution problems using a simple.
from www.youtube.com
When dealing with dilutions such as those in questions 5 and 6, a handy trick can be used. There’s a bottle of 0.750 m nacl on a shelf. If the concentration and volume of the initial solution are c1 and v1. Learn how to dilute and concentrate solutions. How much of it do you need to prepare 50 ml of a. This chemistry video tutorial explains how to solve common dilution problems using a simple. The concentration and the volumes change in a dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. What is the new concentration? Dilution is the addition of.
Dilution Problems Chemistry Tutorial YouTube
Dilution And Concentration Problems A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of. When dealing with dilutions such as those in questions 5 and 6, a handy trick can be used. Learn how to dilute and concentrate solutions. This chemistry video tutorial explains how to solve common dilution problems using a simple. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. How much of it do you need to prepare 50 ml of a. There’s a bottle of 0.750 m nacl on a shelf. What is the new concentration? The concentration and the volumes change in a dilution. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. If the concentration and volume of the initial solution are c1 and v1.
From www.slideserve.com
PPT Volumetric Analysis PowerPoint Presentation, free download ID Dilution And Concentration Problems How much of it do you need to prepare 50 ml of a. What is the new concentration? When dealing with dilutions such as those in questions 5 and 6, a handy trick can be used. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A dilution is a process where. Dilution And Concentration Problems.
From www.youtube.com
Molarity Dilution Practice Problem 2 YouTube Dilution And Concentration Problems Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. Learn how to dilute and concentrate solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. This chemistry video tutorial explains how to solve common dilution problems using a simple. If. Dilution And Concentration Problems.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution And Concentration Problems The concentration and the volumes change in a dilution. Dilution is the addition of. If the concentration and volume of the initial solution are c1 and v1. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. Often, a worker will need to change the concentration of a. Dilution And Concentration Problems.
From www.slideserve.com
PPT Concentration PowerPoint Presentation, free download ID2186169 Dilution And Concentration Problems The concentration and the volumes change in a dilution. What is the new concentration? If the concentration and volume of the initial solution are c1 and v1. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to dilute and concentrate solutions. When dealing with dilutions such as those in questions 5 and. Dilution And Concentration Problems.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Dilution And Concentration Problems Often, a worker will need to change the concentration of a solution by changing the amount of solvent. This chemistry video tutorial explains how to solve common dilution problems using a simple. How much of it do you need to prepare 50 ml of a. A dilution is a process where the concentration of a solution is lowered by adding. Dilution And Concentration Problems.
From www.youtube.com
Solving dilution problems YouTube Dilution And Concentration Problems How much of it do you need to prepare 50 ml of a. Dilution is the addition of. When dealing with dilutions such as those in questions 5 and 6, a handy trick can be used. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. This chemistry video tutorial explains how. Dilution And Concentration Problems.
From www.chegg.com
Solved Dilution Problems For problems 711 use the dilution Dilution And Concentration Problems There’s a bottle of 0.750 m nacl on a shelf. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. This chemistry video tutorial explains how to solve common dilution problems using a simple. If the concentration and volume of the initial solution are c1 and v1. Learn. Dilution And Concentration Problems.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution And Concentration Problems The concentration and the volumes change in a dilution. If the concentration and volume of the initial solution are c1 and v1. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of. This chemistry video tutorial explains how to solve common dilution problems using a simple. How much of it. Dilution And Concentration Problems.
From www.youtube.com
Chem 11 Unit 5 Dilution Calculations Solving for Final Concentration Dilution And Concentration Problems What is the new concentration? A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. This chemistry video tutorial explains how to solve common dilution problems using a simple. How much of it do you need to prepare 50 ml of a. Often, a worker will need to. Dilution And Concentration Problems.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution And Concentration Problems There’s a bottle of 0.750 m nacl on a shelf. When dealing with dilutions such as those in questions 5 and 6, a handy trick can be used. This chemistry video tutorial explains how to solve common dilution problems using a simple. A dilution is a process where the concentration of a solution is lowered by adding solvent to the. Dilution And Concentration Problems.
From materialzonetomlin.z21.web.core.windows.net
Concentration And Dilution Worksheets Dilution And Concentration Problems The concentration and the volumes change in a dilution. Dilution is the addition of. When dealing with dilutions such as those in questions 5 and 6, a handy trick can be used. If the concentration and volume of the initial solution are c1 and v1. A dilution is a process where the concentration of a solution is lowered by adding. Dilution And Concentration Problems.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration Dilution And Concentration Problems If the concentration and volume of the initial solution are c1 and v1. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. Dilution is the addition of. State whether the concentration of a solution is directly or indirectly proportional to its volume. When dealing with dilutions such. Dilution And Concentration Problems.
From www.youtube.com
Dilutions Explained with Problems YouTube Dilution And Concentration Problems A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. When dealing with dilutions such as those in questions 5 and 6, a handy trick can be used. If the concentration and volume of the initial solution are c1 and v1. What is the new concentration? Learn how. Dilution And Concentration Problems.
From www.pinterest.com
DILUTIONS M1V1; M2V2 Solving Dilution Problems in Solution Chemistry Dilution And Concentration Problems When dealing with dilutions such as those in questions 5 and 6, a handy trick can be used. There’s a bottle of 0.750 m nacl on a shelf. What is the new concentration? Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. The concentration and the. Dilution And Concentration Problems.
From www.scribd.com
dilution tutorial and problems Significant Figures Concentration Dilution And Concentration Problems How much of it do you need to prepare 50 ml of a. When dealing with dilutions such as those in questions 5 and 6, a handy trick can be used. This chemistry video tutorial explains how to solve common dilution problems using a simple. There’s a bottle of 0.750 m nacl on a shelf. Learn how to dilute and. Dilution And Concentration Problems.
From www.chegg.com
Solved Dilution Problems For problems 7 11 use the dilution Dilution And Concentration Problems Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute and concentrate solutions. Dilution is the addition of. How much of it do you need to prepare 50 ml of a. There’s a bottle of 0.750 m nacl on a shelf. The concentration and the volumes change in. Dilution And Concentration Problems.
From www.youtube.com
Dilution and Concentration Calculations in Pharmacy 5 Key Examples Dilution And Concentration Problems When dealing with dilutions such as those in questions 5 and 6, a handy trick can be used. If the concentration and volume of the initial solution are c1 and v1. What is the new concentration? A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. State whether. Dilution And Concentration Problems.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution And Concentration Problems Learn how to dilute and concentrate solutions. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. What is the new concentration? How much of it do you need to. Dilution And Concentration Problems.
From www.slideshare.net
Molarity and dilution Dilution And Concentration Problems A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. How much of it do you need to prepare 50 ml of a. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of. There’s a bottle of 0.750. Dilution And Concentration Problems.
From www.studocu.com
Practice Concentration Problems Calculate the Molarity of a solution Dilution And Concentration Problems There’s a bottle of 0.750 m nacl on a shelf. How much of it do you need to prepare 50 ml of a. When dealing with dilutions such as those in questions 5 and 6, a handy trick can be used. Dilution is the addition of. If the concentration and volume of the initial solution are c1 and v1. State. Dilution And Concentration Problems.
From math-physics-problems.fandom.com
Concentration Dilution of Solution Math & Physics Problems Wikia Fandom Dilution And Concentration Problems What is the new concentration? Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. How much of it do you need to prepare 50 ml of a. When dealing with dilutions such as those in questions 5 and 6, a handy trick can be used. If. Dilution And Concentration Problems.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilution And Concentration Problems The concentration and the volumes change in a dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. What is the new concentration? Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. This chemistry video tutorial explains how to solve. Dilution And Concentration Problems.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution And Concentration Problems If the concentration and volume of the initial solution are c1 and v1. This chemistry video tutorial explains how to solve common dilution problems using a simple. The concentration and the volumes change in a dilution. What is the new concentration? There’s a bottle of 0.750 m nacl on a shelf. Often, a worker will need to change the concentration. Dilution And Concentration Problems.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution And Concentration Problems How much of it do you need to prepare 50 ml of a. State whether the concentration of a solution is directly or indirectly proportional to its volume. The concentration and the volumes change in a dilution. Learn how to dilute and concentrate solutions. If the concentration and volume of the initial solution are c1 and v1. This chemistry video. Dilution And Concentration Problems.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution And Concentration Problems A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. What is the new concentration? If the concentration and volume of the initial solution are c1 and v1. Learn how to dilute and concentrate solutions. How much of it do you need to prepare 50 ml of a.. Dilution And Concentration Problems.
From www.youtube.com
How solve dilution and concentration calculation problems YouTube Dilution And Concentration Problems State whether the concentration of a solution is directly or indirectly proportional to its volume. What is the new concentration? The concentration and the volumes change in a dilution. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. This chemistry video tutorial explains how to solve common. Dilution And Concentration Problems.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution And Concentration Problems If the concentration and volume of the initial solution are c1 and v1. Learn how to dilute and concentrate solutions. This chemistry video tutorial explains how to solve common dilution problems using a simple. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. What is the new. Dilution And Concentration Problems.
From www.slideserve.com
PPT Dilution, Concentration and Alligation PowerPoint Presentation Dilution And Concentration Problems Dilution is the addition of. State whether the concentration of a solution is directly or indirectly proportional to its volume. If the concentration and volume of the initial solution are c1 and v1. What is the new concentration? This chemistry video tutorial explains how to solve common dilution problems using a simple. A dilution is a process where the concentration. Dilution And Concentration Problems.
From www.youtube.com
CHEMISTRY 101 Calculating Ion Concentration by Molarity and Solution Dilution And Concentration Problems What is the new concentration? Learn how to dilute and concentrate solutions. How much of it do you need to prepare 50 ml of a. This chemistry video tutorial explains how to solve common dilution problems using a simple. If the concentration and volume of the initial solution are c1 and v1. The concentration and the volumes change in a. Dilution And Concentration Problems.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Dilution And Concentration Problems What is the new concentration? How much of it do you need to prepare 50 ml of a. The concentration and the volumes change in a dilution. Dilution is the addition of. Learn how to dilute and concentrate solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. There’s a bottle of 0.750 m. Dilution And Concentration Problems.
From www.youtube.com
Practice Problems with Solutions, Concentration and Molarity YouTube Dilution And Concentration Problems The concentration and the volumes change in a dilution. There’s a bottle of 0.750 m nacl on a shelf. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. State whether the concentration of a solution is directly or indirectly proportional to its volume. If the concentration. Dilution And Concentration Problems.
From www.chegg.com
Solved Dilution diagrams can be very helpful in organizing Dilution And Concentration Problems When dealing with dilutions such as those in questions 5 and 6, a handy trick can be used. There’s a bottle of 0.750 m nacl on a shelf. What is the new concentration? This chemistry video tutorial explains how to solve common dilution problems using a simple. Often, a worker will need to change the concentration of a solution by. Dilution And Concentration Problems.
From www.youtube.com
Molarity Dilution Practice Problem 3 YouTube Dilution And Concentration Problems The concentration and the volumes change in a dilution. There’s a bottle of 0.750 m nacl on a shelf. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. This chemistry video tutorial explains how to solve common dilution problems using a simple. State whether. Dilution And Concentration Problems.
From studylib.net
Dilutions and concentrations Dilution And Concentration Problems A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more. This chemistry video tutorial explains how to solve common dilution problems using a simple. Dilution is the addition of. State whether the concentration of a solution is directly or indirectly proportional to its volume. There’s a bottle of. Dilution And Concentration Problems.
From www.slideserve.com
PPT Dilutions and concentrations Lab 7 PowerPoint Presentation, free Dilution And Concentration Problems What is the new concentration? Learn how to dilute and concentrate solutions. There’s a bottle of 0.750 m nacl on a shelf. This chemistry video tutorial explains how to solve common dilution problems using a simple. How much of it do you need to prepare 50 ml of a. Often, a worker will need to change the concentration of a. Dilution And Concentration Problems.